New methods for preparing blood components for injection therapy of the locomotor apparatus have experienced an absolute boom over the last ten years. Many different procedures, names, suppliers, and techniques have become established on the market. This explains why it is difficult, not only for practitioners, to keep track of them all. In the literature too, it has now become virtually impossible in the case of many publications to determine with certainty which preparation and which production method were used
to conduct certain studies.
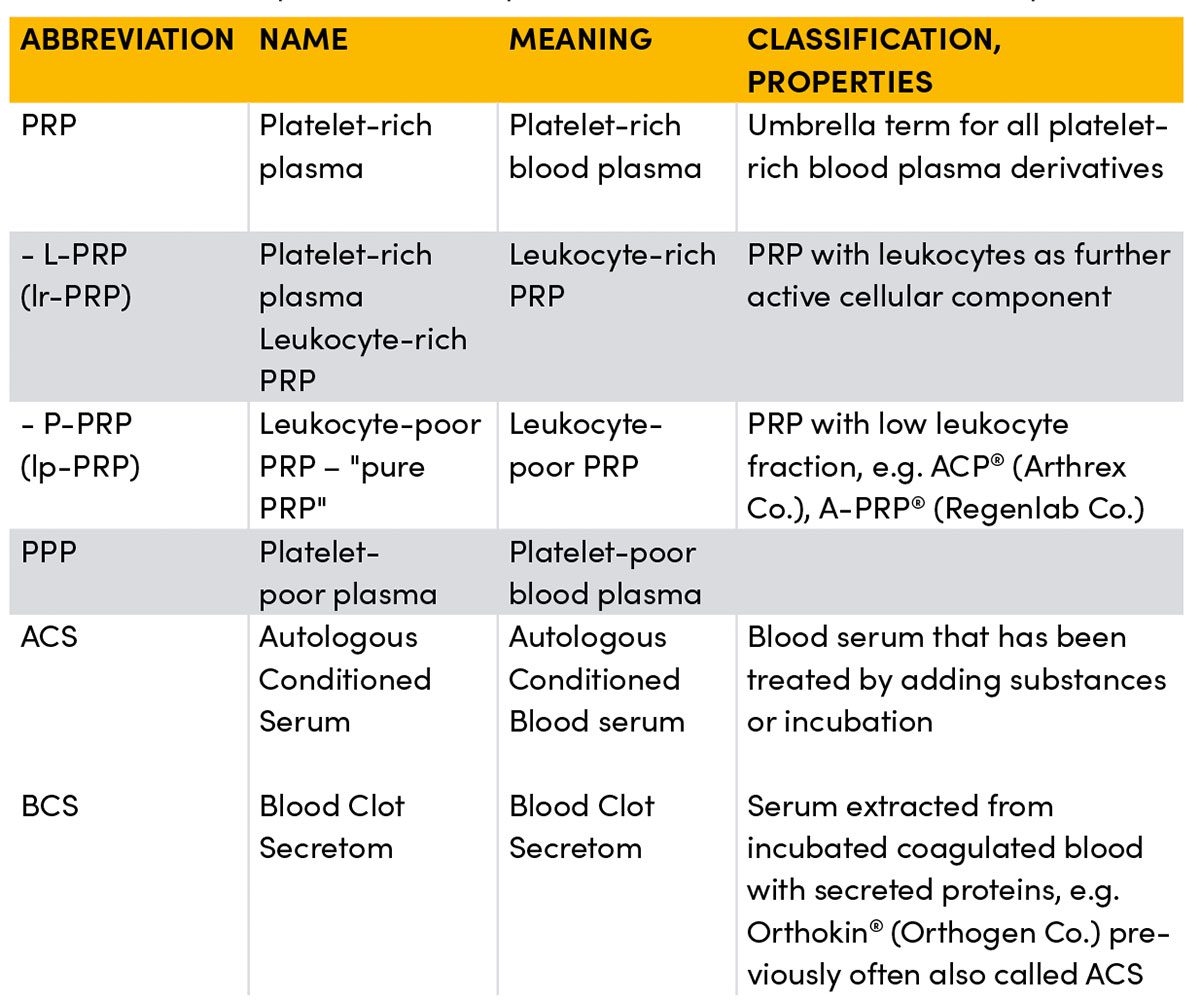
Behind the umbrella term PRP (Platelet-Rich Plasma) alone, probably the best-known application form, hide numerous subgroups that differ, sometimes considerably, regarding the number, duration and speed of centrifugation(s), added constituents, and how they are separated or pipetted, and thus differ in their composition, sometimes considerably.
 Schematic representation of the manufacture of common blood derivatives used in orthopaedics and sports medicine
Schematic representation of the manufacture of common blood derivatives used in orthopaedics and sports medicine
Inconsistent data
Regrettably, the data on file for almost all these procedures are so inconsistent and generally too few for them to be assessed from a scientific standpoint for their use in mass and competitive sports. Although there are now considerable numbers of publications, for example on PRP, the overriding majority, with a few exceptions, are unfortunately of poor quality and most of them have too few participants (often only a total of 20 – 30 patients) to allow any generally valid conclusions to be drawn. Furthermore, the published outcomes are very inconsistent, due first to the fact that there are over 40 established methods and systems world-wide for producing PRP, and second that many studies were conducted using different methods. While almost all the standard procedures for producing PRP on the German market include one centrifugation step, most of the Anglo-American studies include procedures with two centrifugation steps which sharply reduces comparability. In many cases, especially in older studies, the study design makes no mention of which production method was used for the PRP administered. In such a situation, besides the preliminary results from basic and clinical research, we still have to rely on our own empirical findings, the serious exchange of information with experienced colleagues, and last but not least on the feedback from our athletes. Although sports medicine is and will remain an empirical science, it still requires individually optimised treatment for each and every athlete. Nevertheless, without any evidence base at all it is difficult to identify serious, safe and demonstrably effective procedures among the plethora of products and promised cures. For example, there are now very robust and very consistent data on the treatment of osteoarthritis of the knee with blood serum preparations from several level 1 studies (Randomised Controlled Trials, RCTs) that prove the efficacy. The number of studies with positive outcomes on their use for muscular and tendinous injuries is also increasing although they do report considerably more heterogeneous results. Thus it is worth looking more closely at this very popular and commonly used product group and becoming familiar with the classification scheme regarding manufacture, composition and understanding of their presumed mode of action.
PRP (Platelet-Rich Plasma)
So-called platelet-rich plasma is the plasma fraction separated from whole blood in which the platelets are enriched above their concentration in whole blood, usually by centrifugation. In contrast to the US, in Germany there are no specifications prescribing which concentration a procedure must achieve in order to be called a PRP product and be marketed. As a rule though, a concentration of about 2 – 5 times should be gained in order to obtain a therapeutic benefit. During centrifugation the blood components are separated according to their size and weight. In the uppermost layer there is a concentration of plasma (incl. the dissolved proteins) and platelets, but also of cell fragments/components etc. The heavier and larger red blood cells sink to form a deep layer at the bottom. In the intermediate layer between these two fractions a zone forms that contains an increased number of white blood cells, the so-called buffy coat. Depending on whether fractions of this intermediate layer are removed or separated with other fractions this forms leukocyte-poor PRPs (lp-PRP) or leukocyte-rich PRPs (lr-PRP). Equally, the upper layer can be separated again after initial centrifugation. This contains particularly platelet-rich fraction or platelet-poor serum (PPP). Whether the presence of leukocytes in the PRP potentiates the desired effect has long been the subject of controversy and has certainly not been scientifically established. A growing number of studies are indicating that leukocyte-rich PRP is particularly effective for insertion tendinosis, for example in lateral epicondylopathy at the elbow. In contrast, other papers report that a high concentration of immune cells promotes a catabolic and inflammatory metabolic status which, for example, could have a negative effect on wound healing processes. The overview is further complicated by the fact that treating the blood before centrifugation has an effect on the composition of the subsequent preparation. For instance, untreated autologous blood can be centrifuged as in, for example, ACP® (Autologous Conditioned Plasma) from the Arthrex Co., or conditioned, for instance, by an incubation phase or by adding bioactive substances such as citrate, EDTA, or certain cytokines. This is then called autologous conditioned serum (ACS).
ACS (Autologous Conditioned Serum)
Pure plasma products such as Orthokin® from the Orthogen Co. must be classified separately from the platelet-rich plasma products. When manufactured properly this infiltrate ultimately no longer contains any cells, neither platelets nor immune cells, but only their concentrated secretion proteins produced by incubation and centrifugation (mostly cytokines). This is why these preparations are now also called blood clot secretom (BCS). Thus, when injected later, no relevant amounts of living cells or cell fragments are introduced at the injection site. Whether or not this is desirable depends on the respective indication. Overloaded or injured tissue is usually in a state of stress that is associated with altered tissue homoeostasis, i.e. pH, oxygen concentration, energy production and the presence of immune cells. However, hardly any research has been conducted to date on the effect of administering larger numbers of living cells into this milieu that are metabolically active and thus require energy and oxygen, and following apoptosis themselves have to be transported away from the site, phagocytosed and metabolised. This fact may be one of many possible explanations of why PRP injections for sports injuries, and above all the consequences of overstrain, deliver such heterogeneous results. The desired positive effect of the injected platelets, and in some cases immune cells, might depend strongly on whether their type and concentration are suitable for the current tissue milieu at the injection site where they trigger a beneficial, neutral or more disadvantageous effect. Since there have not been any clinically applicable measuring methods for this to date and the concentration of cell components can fluctuate widely from time to time within a patient, even with the same preparation, this is largely foreseeable. In contrast, when using cell-rich plasma preparations, especially for larger tissue defects such as tendon or ligament ruptures, fractures etc., one often sees the accelerated formation of a load-bearing scar. It is plausible that the cell clot that forms in the injured area after a PRP injection can stimulate connective tissue healing over a very much longer period of time, and that the associated filling of the tissue defect with fibrin, proteins and cells could represent a matrix for reorganisation of the scar tissue.
Summary
Thus the benefits and disadvantages of different preparations and in turn their indications or relative contraindications depend to a large extent not only on the patient’s situation, the type, scope and localisation of the injury, but also on the experience and knowledge of the respective practitioner. From practical experience it can be generally stated that the use of one group of preparations alone for all musculoskeletal indications is certainly not ideal. It is the job of researchers, but also of practitioners, to collect experience with the uses, outcomes and further courses after using the individual preparations, and to exchange these experiences frankly and seriously. Only in this way can we manage to select the right preparations for our patient from the booming range of different preparations, and thus ensure that the reliability and acceptance of these methods increase further among athletes, colleagues and health insurance agencies.

left recent medial ligament rupture with only little dehiscence (blue arrow) and scarred thickening of the ligament (orange arrow) after an old previous injury; middle and right: progressive connective tissue repair at the rupture zone (blue arrows) without significant thickening of the ligament and remodelling of the distal scar area, in this case after 4 BCS injections (Orthokin®, Orthogen Co.) and adapted early functional exercises. Follow-up MRI after 3 and 6 weeks

left distal myotendinous injury of the biceps femoris muscle with a pronounced structural defect; right: homogeneous scarred healing of the defect area 6 weeks later after 3 applications of intra- and perilesional PRP (ACP®, (Arthrex Co.).
The author has the references
Autoren
ist Facharzt für Orthopädie und Unfallchirurgie. Er ist seit 2018 festangestellter Mannschaftsarzt von Borussia Mönchengladbach, zusätzlich arbeitet er im Orthopädiezentrum Theresie in München. Zuvor war Dr. Doyscher in verschiedenen Abteilungen der Charité Berlin tätig. Außerdem war er Mannschaftarzt des 1. FC Union Berlin sowie Verbandsarzt des DLV und BSD. Dr. Doyscher ist wiss. Beirat der sportärztezeitung.
ist Facharzt für Orthopädie und Unfallchirurgie und arbeitet in der ATOS-Klinik München. Seit 2002 ist er Mannschaftsarzt der Deutschen Ski-Nationalmannschaft alpin und Verbandsarzt des Deutschen Skiverbandes (DSV), seit 2015 leitender Mannschaftsarzt des DSV. Er ist Kniespezialist mit dem Schwerpunkt auf regenerative und rekonstruktive Verfahren sowie Muskel-Sehnenverletzungen an der unteren Extremität.