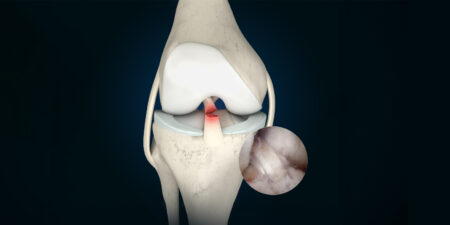
With roughly 27 million ankle injuries worldwide each year, and roughly 1.7 million associated osteochondral lesions of the talus (Baumhauer et al. Am J Sports Med. 1995; 23 (5): 564 – 570), potentially with consequential damage, the orthopedic and trauma surgery community has some considerable interest in improving post-op results over the long term.
On the one hand, ankle trauma can cause changes in the sense of bone marrow edema (bone bruise) due to the mechanical aspect of the mode of injury; on the other hand, injuries to the cartilage of the talus (chondral lesions) or combined bone-cartilage injuries (osteochondral lesions) can also occur as a result of the trauma. Osteochondral lesions of the talus can be accompanied by subchondral cysts. Patients affected complain of ankle pain, some of which is unrelated to pressure being applied, of restricted ankle movement, some of swelling and locking-up, as well as instances of functional instability [4]. In addition to the symptoms described above, clinical examination of the patient may reveal unstable syndesmoses, problems in the area of the medial and lateral capsular ligament apparatus, and axial deviations in the hindfoot, requiring further treatment [5].
Treatment options up till now have involved conservative and surgical measures. Conservative measures include relief using forearm crutches, taking NSAIDs, physiotherapy exercise, providing ankle orthoses and shoe insoles, and perhaps intra-articular corticosteroid injections. Surgical treatment options are roughly divided into bone marrow stimulation, cartilage repair and cartilage regeneration measures. In recent years, injections with hyaluronic acid preparations and platelet rich plasma (PRP) preparations have been attracting more and more attention in the treatment of osteochondral lesions of the talus. Various studies have demonstrated improved joint mobility and reduced post-op pain where growth factors and bioactive components, contained in the PRP, were injected intraoperatively during ankle operations. Interestingly, these studies have shown that the improvement in the above factors is independent of the surgical technique [3]. Below, we discuss some new techniques for treating bone marrow edema, and chondral and osteochondral defects of the talus.

Biological basis
As already described above, use of PRP for this type of injury has become more prominent in the last ten years due to the positive outcomes achieved. To better understand the effect of PRP, it is necessary to look more closely at the physiology of tissue regeneration. The healing mechanism generally applies in equal measure to all tissue types and following haemostasis is in three phases:
Inflammatory response
Platelets and leukocytes in the blood clot release growth factors and numerous other cytokines, which trigger the inflammatory response. Endothelial cells also present then guide the inflammatory processes to the injury site [7]. Complex metabolic cycles involving neutrophils and macrophages, amongst others, ensue [8]. The latter, activated by messenger substances from leukocytes, then initiate the release of healing factors such as TGF-ß, bFGF, PDGF and VEGF [9].
Proliferation
Released VEGF stimulates plasma proteins to lay down a provisional matrix, into which stromal progenitor cells, guided by cytokines released by the immune cells, then grow. The progenitor cells differentiate depending on the growth factors and cytokines present, developing into the predominant tissue-specific cell type (approx. 3 – 5 days after injury). Stimulated by PDGF, IGF and TGF ß, these cells also produce collagen, proteoglycans and other components of the extracellular matrix [10].
Remodelling
Collagen deposition peaks approx. 2 – 3 weeks after injury and the transition to the remodelling phase begins. A balance develops between synthesis, accumulation and degradation. Small capillaries join to form larger vessels. Water content, cell density and metabolic activity fall. The type, quantity and organisation of collagen change significantly, resulting in increased firmness. The initially deposited collagen III becomes collagen I, and the physiological ratio of 4:1 (collagen 1 to 3) is restored [11]. If this physiological healing cascade is disturbed at some point by intrinsic or extrinsic factors, various pathologies result depending on severity and tissue type.
Platelet-Rich Plasma – PRP
PRP, usually produced from whole blood by centrifugation, features a greater concentration of platelets and so also greater concentration of the growth factors it contains. When PRP is injected into the affected region, the greater concentration of growth factor has a positive effect on cell proliferation, differentiation, chemotaxis, and angiogenesis [11]. Impaired healing processes start up again and affected tissue is stimulated into recovery.
Use in Bone Regeneration
Like in other tissues, PRP has positive effects on cell proliferation, differentiation, chemotaxis, and angiogenesis in bone healing. With platelets having a lifespan of 7 – 10 days, it is assumed that PRP supports early bone healing rather than influencing late bone formation [12]. There is increasing evidence that platelet-induced inflammation response plays a major role in the early stages of recovery, and that effective regeneration cannot occur without it [12]. Excessive inflammation, however, can have a negative effect on recovery. Here, PRP has a positive effect on both the extent and duration of the inflammation, through the growth factors TGF-β1, IL-4, HGF and TNF-α [12], such that recovery is channeled in the right direction at an early stage.
Use with Cartilage Damage
Cartilage has only very limited self-healing capacity when damaged, due to its inherent avascularity, which therefore leads to cartilage damage and osteoarthritis. However, numerous growth factors play a central role in the development and homeostasis of cartilage, suggesting the use of PRP in cartilage regeneration. Anabolic factors, such as TGF-ß1 or IGF-I, stimulate chondrocytes into synthesizing proteoglycans, aggrecan and collagen II. They induce proliferation of synoviocytes and mesenchymal stem cells. At the same time, the catabolic effects of, for example, Interleukin 1 (IL-1) or matrix metalloproteinases (MMPs) are reduced [13].
Bone Marrow Edema of the Talus
Bone marrow edema is a pathological increase in interstitial fluid in the bone, and can be detected early using an MRI scan, if there is vague joint pain (Fig. 2). There are various causes of bone marrow edema. Persistent joint pain is called bone marrow edema syndrome (BMES), which is defined as lasting from 3 to 18 months [14 – 16]. It is worth mentioning that distinguishing it from osteonecrosis of the talus can be difficult. As a rule, however, osteonecrosis follows a fulminant disease progression. Bone marrow edema can roughly be classified as vascular ischemic, mechanical or traumatic, and reactive bone marrow edema. Bone marrow edema of the talus is often visible on an MRI scan following trauma. How and why bone marrow edema syndrome develops from bone marrow edema remains unclear [17].

Clinical Symptoms
Clinical symptoms manifest themselves as acute pain and significant impairment of function, and swelling in or on the ankle. There are normally no signs of local inflammation.
Imaging Procedures
Diffuse osteopenia can sometimes be seen in the affected area on unenhanced x-rays. Bone scans with detectable tracer accumulation in bone marrow edema are an indication of increased bone regeneration activity. If it is uniform, the surrounding soft tissues are not affected. Sensitivity is around 60 % [18]. Magnetic resonance imaging is the method of choice for confirming the diagnosis. MRI scans have 100 % sensitivity. In order to differentiate osteonecrosis from bone marrow edema, using a gadolinium-based contrast agent is recommended [19].
Therapy
Basically, a conservative management approach is preferable. Treating the symptoms, taking pressure off the side affected, and taking anti-inflammatory drugs, as well as manual therapy and physiotherapy should be tried first. Recent studies show that IV administration of iloprost or bisphosphonates (such as ibandronate) can lead to a significant improvement in symptoms. Administration of the drugs mentioned above is intended to improve blood circulation (iloprost) or to inhibit osteoclasts (bisphosphonates) [20 – 23]. It is vital to pay attention to the adverse drug reactions which can occur with IV administration of iloprost and bisphosphonates. In particular, localized osteonecrosis of the jaw and atypical femoral fractures should be mentioned, especially when bisphosphonates are being administered [26].
During surgical procedures, the affected bone area is drilled (core decompression) [24, 25]. Symptoms can be improved through the reduction in pressure that results from drilling, as the pain is reduced. It is furthermore assumed that drilling can lead to increased blood flow or even revascularization. The effect of PRP on bone healing has already been described in the biological basis. This also applies to bone marrow oedema. Like in other tissues, PRP has positive effects on cell proliferation, differentiation, chemotaxis, and angiogenesis in bone healing. These connections lead us to a new therapeutic approach. In addition to the pressure relief from surgery described above, by drilling into the bone, platelet-rich fibrin (PRF) made from PRP and autologous thrombin solution is also infused into the affected bone areas. Only material from the patient themselves is used to produce the PRF.
Producing the biologic substances (see Fig. 3): PRP (ACP Autologous Conditioned Plasma). 15 ml ACP can be produced from 45 ml of venous blood, using 3 ACP double syringes (Arthrex GmbH). Autologous thrombin solution: The Thrombinator System (Arthrex GmbH) is used to produce the thrombin solution. The Thrombinator process uses the blood-clotting cascade mechanism to produce an autologous thrombin serum, avoiding the use of aggressive chemical reagents such as ethanol. The design of the Thrombinator eliminates the need for prolonged incubation times and heating. The autologous thrombin solution is produced from PRP in roughly 15 minutes directly at the point of use.

Application of Biologic Substances in Bone Marrow Edema
This can be performed as a minimally invasive surgical procedure (arthroscopic). With the retrograde drilling, placing a targeting tool intra-articularly over the affected cartilage-bone areas is recommended, in order to allow drilling in a targeted manner. If necessary, intraoperative X-rays can be used for checking. First, retrograde drilling using the targeting tool (GPS system, Arthrex GmbH) (Fig. 4 + 5). Platelet-rich fibrin is then infused into the hole using a tapered inserter. The taper prevents the PRF from flowing back before gelling when pressed gently into the hole. Finally, the blind hole is sealed up using bone filler (INNOTERE Paste-CPC, Arthrex GmbH). Platelet-rich fibrin is prepared by mixing PRP and autologous thrombin solution, simulating the final step in the coagulation cascade, in which a stable amount of fibrin is produced from fibrinogen (contained in PRP) and thrombin. Patients use walkers and forearm crutches to relieve pressure post-op until the wound has healed. Full weight bearing is usually possible immediately once the wound has healed, provided there has been no surgery on associated injuries. In one observational study, patients showed pain reduction using the visual analogue scale, from an average of VAS 9 to VAS 1, 14 days after the combined surgical procedure of drilling the bone and infusing PRF intraosseously.

Chondral Lesions and Osteochondral Lesions of the Talus
Chondral or osteochondral lesions of the talus occur particularly in young patients as a result of trauma, in most cases sprain trauma. Flick and Gould’s studies of 500 recorded cartilage-bone injuries to the talus show a distribution of 98 % lateral talar dome lesions and 70 % medial talar dome lesions. The authors were able to demonstrate that the causes can be acute trauma as well as repetitive microtraumas affecting talar cartilage.
Clinical Symptoms
The focus is again on ankle pain, which shows no improvement even after prolonged immobilization or physical therapy measures. The pain indication is localized either medially or laterally, some patients reporting feelings of locking-up, and there may be clinically significant swelling and effusion in the ankle joint. The medical history almost always reveals an accident that occurred only recently.
Imaging Procedures
Imaging procedures include unenhanced X-rays in 3 planes under load (AP mortise view) [27]. If there are specific uncertainties concerning assessment of the hindfoot, a Salzmann image may be needed as well. CT scans are particularly important where bony structures are involved, and help to determine the depth of the lesion [28, 29]. For some time now, digital volume tomography has been used to obtain three-dimensional images of the ankle with pressure applied. These images with pressure applied have the advantage that treatment planning is more precise, since the bone position changes in situations with pressure applied. Standard procedures include MRI scans, which can map articular cartilage using cartilage-sensitive pulse sequences. It is possible to see changes in cartilage in the MRI scan, as well as changes in subchondral bone, which cannot be detected in normal X-rays. The sensitivity and specificity for cartilage changes in the talus is given as 96 % [30]. dGEMRIC sequences allow us to measure directly the concentration of GAG (glycosaminoglycan). However, this procedure requires intravenous injection of gadolinium-based contrast agents. In recent years, the so-called SPECT procedure has gained popularity [31]. SPECT stands for Single Photon Emission Computed Tomography, and is a special type of CT scan used to differentiate between active lesions of the cartilage bone complex on the talus, and inactive cartilage bone lesions on the talus.
Treatment
Treating the chondral lesion on the talus depends on the size of the cartilage damage. Cartilage damage smaller than 1 cm² and less than 5 mm thick is usually treated with microfracture or nanofracture (bone marrow stimulation) [27]. Cartilage damage larger than 1.5 cm is usually treated using autogenous bone graft techniques or, if possible, autologous chondrocyte implantation [5]. Refixation can be carried out for intact, chondral fragments at least 3 mm thick. Refixation is carried out in such cases using biodegradable compression screws, darts or pins [27]. Treatment of osteochondral lesions usually involves both building up the damaged bone area and treating the affected cartilage area. Cartilage has only very limited self-healing capacity when damaged, due to its inherent avascularity, which can therefore lead to osteoarthritis. Here, too, the thinking is to combine the surgical procedure with PRP and thrombin.
Surgical Procedure
With chondral lesions, care should be taken to ensure that the cartilage defect is debrided and prepared appropriately. Look out for clean, healthy cartilage edges.
AutoCart™ Procedure (Fig. 6 – 9)
Chondral fragments are harvested from the cartilage margin using a 3 mm shaver (Sabre 3 mm, Arthrex GmbH). Alternatively, cartilage chips can also be removed from non-load-bearing areas on the knee as required. Fragments are harvested in a GraftNet tissue collector (Arthrex GmbH) and then transferred to a 1 ml syringe with a Luer lock connection. The chondral fragments are then mixed with PRP in a ratio of 3:1 using a female to female adapter. On the one hand, this creates a homogeneous paste-like mass, and on the other hand, the ACP contains the fibrinogen needed for clotting. The 1 ml syringe is connected to the application cannula and the fragments transferred into the cannula. The fragments are then carefully pushed to the cannula tip using the cannula trocar, until they appear in the opening. The arthroscopic fluid should then be drained from the ankle joint and the lesion dried as much as possible. The fragments mixture is now carefully pushed forward with the trocar and applied into the defect. The fragment paste is then carefully covered drop by drop with the prepared thrombin serum. The Thrombinator method relies on the blood clotting cascade mechanism. The combination of the fibrinogen contained in the paste and the thrombin applied creates a stable clot that holds the mixture in place in the lesion. For the seal, mix the PRP with thrombin in the ratio 1:1. After mixing, apply the mixture quickly to the lesion drop by drop. Then wait about 2 minutes. The joint should be carefully moved under visual control, to check the congruence of the joint components.

Fig. 6 Shaver blade with GraftNet adapter 
Fig. 7 Harvesting cartilage using a shaver blade 
Fig. 8 Applying the cartilage paste 
Fig. 9 Sealed with PRF (Platelet-rich fibrin) 
Fig. 10 Chondral lesion following preparation 
Fig. 11 Chondral lesion after filling with AutoCart 
Fig. 12 Osteochondral lesion following spongiosaplasty 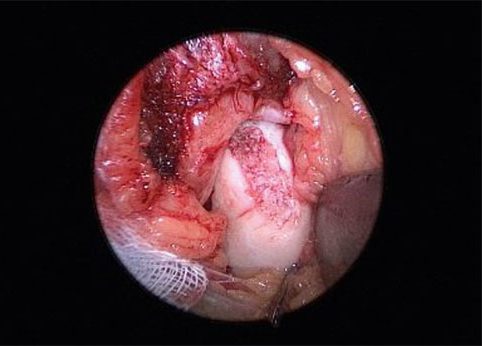
Fig. 13 Osteochondral lesion after filling with AutoCart
Figures 10 and 11 show intra-operative images of a chondral lesion being treated using AutoCart. When treating osteochondral lesions, the stable clot mentioned earlier, consisting of fragmented cartilage and PRF, is placed and fixed onto the spongiosaplasty after surgical reconstruction (Fig. 12 + 13). Here, too, the joint should also be carefully moved under visual control following infusion, to check the congruence of the joint components. The patient uses a walker with 20 kg partial weight load for six weeks post-op. A splint with a 20-0-20 dorsiflexion/plantar flexion range of motion is recommended. Lymphatic drainage and, if necessary, analgesia should be prescribed. NSAIDs should be avoided due to their fibrocystinhibiting effect. From the 7th week, depending on the clinical picture, more pressure can start to be applied. In an observational study, both procedures (arthroscopic as well as open with spongiosaplasty) proved to be safe, at least in the short term, and easy to carry out. Long-term results will show how tissue regeneration is progressing.
Literatur
[1] Gianakos AL, Yasui Y, Hannon CP, Kennedy JG. Current management of talar os- teochondral lesions. World J Orthoped. 2017;8(1):12 – 20.
[2] Shimozono Y, Yasui Y, Ross AW, Kennedy JG. Osteochondral lesions of the talus in the athlete: up to date review. Curr Rev Muscoskel Med. 2017;10(1):131 – 140.
[3] Yausep OE, Madhi I, Trigkilidas D, JOO, Platelet rich plasma for treatment of osteochondral lesions of the talus. J of Orthop., 18(2020) 218 – 225
[4] Gökay Görmeli, MD1, Mustafa Karakaplan, MD1, Cemile Ayşe Görmeli, MD2, Baran Sarıkaya, MD3, Nurzat Elmalı, MD4, and Yüksel Ersoy, MD5, Clinical Effects of Platelet-Rich Plasma and Hyaluronic Acid as an Additional Therapy for Talar Osteochondral Lesions Treated with Microfracture Surgery: A Prospective Randomized Clinical Trial. Foot & Ankle International® 2015, Vol. 36(8) 891–900
[5] Eoghan T. Hurley, MB, BCh1, Christopher D. Murawski, MD2, Jochen Paul, MD3, Alberto Marangon, MD4, Marcelo P. Prado, MD5, Xiangyang Xu, MD6, Laszlo Hangody, MD7,8, John G. Kennedy, MD, MCh, MMSc, FRCS (Orth)9, and the International Consensus Group on Cartilage Repair of the Ankle, Osteochondral Autograft: Proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot & Ankle International®2018, Vol. 39(1S) 28S–34S
[6] Ahmed Aly Elghawy, Carlos Sesin, Michael Rosselli, Osteochondral defects of the talus with a focus on platelet-rich plasma as a potential treatment option: a review. BMJ Open Sport & Exercise Medicine 2018;4:e000318. doi:10.1136/
[7] Verhamme P, Hoylaerts MF: Hemostasis and inflammation: Two of a kind? Thromb J 7:15, 2009
[8] Borregaard N, Sørensen OE, Theilgaard-Wnchl K: Neutrophil granules: A library of innate immunity proteins.Trends Immunol 28:340-345, 2007
[9] Krysko DV, D’Herde K, Vandenabeele P: Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 11:1709-1726, 2006
[10] Magra M, Maffulli N: Matrix metalloproteases: A role in overuse tendinopathies. Br J Sports Med 39:189-191, 2005
[11] Andia I, Sánchez M, Maffulli N: Basic Science: Molecular and Biological Aspects of Platelet-Rich Plasma Therapies, Operative Technique in Orthopedics, 22:3 – 9 © 2012
[12] Oryan A, Alidadi, Moshiri A: Platelet-rich plasma for bone healing and regeneration Expert Opinion on biological therapy, 2016
[13] Fortier L, Barker J, Strauss E.J, McCarrel T, Cole B: The role of growth factors in cartilage repair, Clin. Orthop Relat Res, DOI 10.1007/s1 1999-011-1857-3
[14] Aigner N, Maizer R, Meraner D et al (2009) Bone marrow edema syn- drome in postpartal women: tre- atment with iloprost. Orthop Clin North Am 40(2):241 – 247
[15] Guerra JJ, Steinberg ME (1995) Dis- tinguishing transient osteoporosis from avascular necrosis of the hip. JBone Joint Surg Am 77(4):616–624
[16] Disch AC, Matziolis G, Perka C (2005) The management of necro- sis-associated and idiopathic bone- marrow oedema of the proximal fe- mur by intravenous iloprost. J Bone Joint Surg Br 87(4):560–564
[17] Plenk H Jr, Hofmann S, Eschberger J et al (1997) Histomorphology and bone morphometry of the bone marrow edema syndrome of the hip. Clin Orthop Relat Res 334:73–84
[18] Lai K-A, Shen WJ, Yang CY et al (2005) The use of alendronate to prevent early collapse of the femo- ral head in patients with nontrau- matic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am87(10):2155–2159
[19] Thiryayi WA, Thiryayi SA, Freemont AJ (2008) Histopathological per- spective on bone marrow oede- ma, reactive bone change and hae- morrhage. Eur J Radiol 67(1):62–67
[20] Meizer R, Radda C, Stolz G et al (2005) MRI-controlled analysis of 104 patients with painful bone marrow edema in different joint lo- calizations treated with the prosta- cyclin analogue iloprost. Wien KlinWochenschr 117(7–8):278–286
[21] Aigner N, Petje G, Schneider W et al (2005) Bone marrow edema syn- drome of the femoral head: treat- ment with the prostacyclin analo- gue iloprost vs. core decompressi- on: an MRI-controlled study. Wienin Wochenschr 117(4):130–135
[22] Baier C, Schaumburger J, Götz J et al (2012) Bisphosphonates or pro- stacycline in the treatment of bo- ne-marrow oedema syndrome of the knee and foot. Rheum Int Nov10
[23] Radke S, Kirschner S, Seipel V et al (2003) Treatment of transient bo- ne marrow oedema of the hip – a comparative study. Int Orthop27(3):149–152
[24] Plenk H Jr, Hofmann S, Eschberger J et al (1997) Histomorphology and bone morphometry of the bone marrow edema syndrome of the hip. Clin Orthop Relat Res 334:73–84
[25] Wilson AJ, Murphy WA, Hardy DC, Totty WG (1988) Transient osteoporosis: transient bone mar- row edema? Radiology 167(3):757–760
[26] www.aerzteblatt/archiv/53506
[27] Charles P. Hannon, MD‘, Steve Bayer, BA7, Christopher D. Murawski, MD, Gian Luigi Canata, MD, Thomas O. Clanton, MD‘, Daniel Haverkamp, MD, PhD’, Jin WooLee, MD, PhD‘, Martin J. O’Malley, MD’, Hua Yinghui, MD, PhD®,James W. Stone, MD? and the International Consensus Groupon Cartilage Repair of the Ankle Debridement, Curettage, and Bone Marrow Stimulation: Proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle Foot & Ankle Internationak 2018, Vol. 39(1S) 165-22$
[28] Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc 2010; 18:419-433 [PMID: 20119671 DOI: 10.1007/s00167-010-1054-z]
[29] Nakasa T, Adachi N, Kato T, Ochi M. Appearance of Subchondral Bone in Computed Tomography Is Related to Cartilage Damage in Osteochondral Lesions of the Talar Dome. Foot Ankle Int 2014; 35: 600-606 [PMID: 24677221 DOI: 10.1177/1071100714528493]
[30] Verhagen RA, Maas M, Dijkgraaf MG, Tol JL, Krips R, van Dijk CN. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br 2005; 87: 41-46 [PMID: 15686236]
[31] Meftah M, Katchis SD, Scharf SC, Mintz DN, Klein DA, Weiner LS. SPECT/CT in the management of osteochondral lesions of the talus. Foot Ankle Int 2011; 32: 233-238 [PMID: 21477540 DOI: 10.3113/FAI.2011.0233]
Autoren
ist seit 2005 Gesellschafter im sporthopaedicum. Sein Schwerpunkt ist die konservative und operative Versorgung von Erkrankungen und Verletzungen des Sprunggelenkes und Fußes. Zudem beschäftigt er sich intensiv mit der Behandlung von Sportverletzungen und ist Mitglied im Vorstand der Gesellschaft für Arthroskopie und offene Gelenkchirurgie (AGA).