Treatments of tendinopathies and other indications of the musculoskeletal system with individual modalities (e. g., a certain drug or a certain device) are cost-effective and justified in case of non-athletes. However, in case of athletes (and particularly in case of professional athletes) such treatments are usually not justified for several reasons. One reason is that combination therapy may address different molecular and cellular target pathways, resulting in better healing, accelerated return to sport and improved prevention of disease or injury recurrence. Another reason is that an individual subject may not respond to a certain treatment modality but to another, and consecutive application of these treatment modalities could result in prolonged healing and delay in return
to sport. Here we report successful treatment of two athletes with a novel combination of radial extracorporeal shock wave therapy and neuroreflectory hyperbaric CO2 cryotherapy.
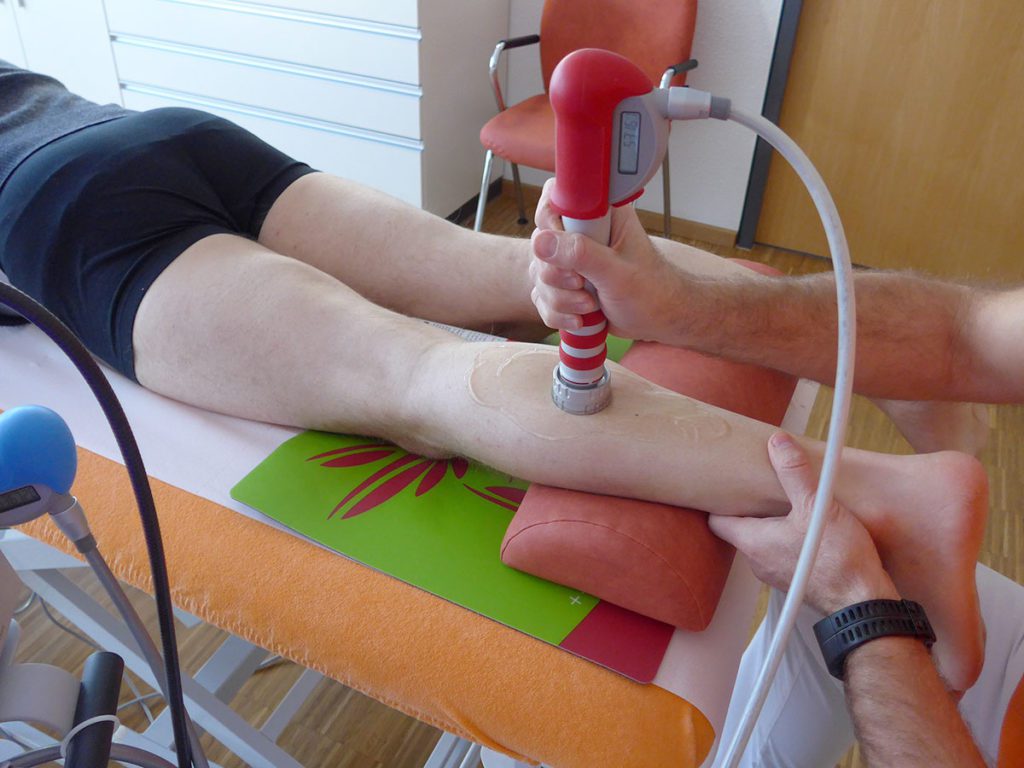
Radial extracorporeal shock wave therapy (rESWT) was performed with a Swiss DolorClast device and EvoBlue + Power Plus handpiece (Electro Medical Systems S.A., Nyon, Switzerland), and neuroreflectory hyperbaric CO2 cryotherapy (NHCC) with a Cryolight device (ELMAKO, Iffezheim, Germany).


Case 1: 18-year-old male German Bundesliga youth handball player,five years competitive sports
Patient’s history recurrent with increased strain on both legs since 1.5 years (increased sport and handball intensity); movement and strain pain under the patella on both sides (pain score on the visual analog scale (VAS) from 0 (no pain) to 10 (maximum pain): 6–7); in resting phases pain in regression; physiotherapy performed with temporary pain reduction; continuation of active handball playing with tape bandage despite pain. Three weeks before the first consultation of Dr. René Toussaint (RT) pain already increased during normal walking, climbing stairs and going down; fast walking and jumping very painful; no significant side difference.
Clinical findings (at the time of first consultation of RT) gait pattern disturbance right; 1-toe position possible, but unsafe on right side; local: distinct pressure pain and slight edema in the base region at the lower patellar pole (right > left); reduced patella mobility; isometric pain during knee extension on both sides; no effusion; no ligament instability; no meniscus signs; positive flexion / extension 140 / 0 / 0 on both sides; movement pain; tightly end feeling for flexion on both sides; distinct muscular imbalances with shortening of the front and rear muscle chains of both legs; strength imbalance between knee flexors and extensors on both sides to the disadvantage of knee extensors.
Imaging diagnostics (already performed before consultation of RT) x-ray, sonography, MRI.
Diagnosis patellar tip syndrome on both sides (jumper´s knee; insertion tendopathy of the M. rectus femoris).
Therapy six therapy sessions in total; initial series of 3 therapy sessions each three to four days apart, then therapy-free interval of three weeks, followed by another series of 3 therapy sessions each three to four days apart; rESWT: 5000 radial extracorporeal shock waves (rESWs) per session and side; rESWs generated with the 15-mm applicator and 2 bar @ 15 Hz; rESWT and NHCC applied to the tip of the patella and the patellar ligament on both sides; in addition, NHCC combined with stretching of the M. quadriceps femoris on both sides.
Supportive therapy sport physiotherapeutic intervention at irregular intervals; wearing of sensorimotor insoles in everyday life.
Additional own activities by the athlete eccentric training; muscle training (strengthening, stretching); local ice treatment at irregular intervals; ointment bandages at night.
Time course significant reduction in pain on both sides (VAS 2) after the first series of therapy sessions; no sports leave or modification of sports load possible; increase in stress pain after three weeks due to high sports load (handball) (VAS 4–5), then decision to perform another series of 3 therapy sessions; markedly reduced pain under stress (right knee: VAS 0–1; left knee VAS: 1–2) after the second series of therapy sessions; continuation of sports; last check-up six weeks after the end of the second series of therapy sessions, with full fitness for sport restored and no complaints (especially no pain during exercise).
Case 2: 55-year-old male marathon runner (leisure sport in running group)
Patient’s history ten days before first consultation of RT strain pain in the left calf after a longer run (approximately 15 km); increase in pain 3 days later on the basis of continued running training; afterwards rest pain even without walking load and increasing stretching pain in the left calf in everyday life; extension of the pain symptoms to the entire calf; no subjective muscle weakness in the leg; then pain enhancement in the left calf but also in left thighs already during normal walking as well as climbing and walking down stairs; in addition, swelling of the left lower leg and ankle joint after daily strain.
Clinical findings gait pattern disorder with relief limps on the left side; 1-toe position painfully reduced on the left side; insecure, heel-toe position / gait convertible, but with pain on the left side (lower leg dorsally); distinct pressure pain M. gastrocnemius (middle and distal parts, predominantly mediolaterally (deep) and laterally); strand-like structure palpable without gap or hematoma; calf circumference not increased; regular function of adjoining joints, except of pain during forced maximum dorsal extension of the left foot; muscular dysbalances with distinct shortening of the posterior muscle chain in both upper and lower leg (left > right); no impairment of blood circulation, muscle strength and sensitivity; slight pain-related impairment of muscle strength during plantar flexion of the left foot (Grade 5- according to Janda); no isometric pain; no clinical evidence of Achilles tendon pathology.
Imaging diagnostics exclusion of thrombosis and structural damage (muscle fibre tear) by sonography and MRI.
Diagnosis pulled muscle left M. triceps surae; myofascial trigger points left lateral M. gastrocnemius and left M. soleus.
Therapy six therapy sessions in total; therapy sessions each three to four days apart; rESWT: 5000 rESWs per session; rESWs generated with the 15-mm and 36-mm applicators and 1.6–3.5 bar @ 15 Hz; rESWT applied to the entire left
M. gastrocnemius; NHCC combined with stretching of the left M. gastrocnemius and left M. soleus; kinesiotaping of the left calf.
Supportive therapy physiotherapeutic treatment in the course of gait and running training with dosed, pain-adapted load adjustment.
Additional own activities by the athlete muscle training (stretching); fascia role; compression stockings (Class 2).
Time course significant reduction of pain after the first and second therapy sessions (VAS 2); no running training but modification of strain (cycling, swimming, aqua jogging) to accompany therapy; control examination 3 weeks after the end of therapy: full sporting ability restored; no complaints, in particular no pain in the left calf in everyday life and during a smooth test run, recommendation for gradual build-up of strain; no follow-up visit because of this injury.

Background
The molecular and cellular mechanisms of ESWT on the musculoskeletal system are pretty well understood. Intensive basic research over the last 20 years demonstrated that neurogenic inflammation contributes to the pathology of tendinopathies [1], and ESWs are thought to play a role in the blockade of neurogenic inflammation [2]. Besides this, exposure of tendons to rESWs can stimulate tendon remodelling in tendinopathy by promoting inflammatory and catabolic processes that are associated with removing damaged matrix constituents [3]. Then, induction of the proliferation of fibroblasts [4] may ultimately induce healing. Besides this, a variety of mechanisms of ESWs on muscles were demonstrated in the literature, including selective impairment of end plates at neuromuscular junctions (caused by impairment of acetylcholine receptors) [5], functional angiogenesis / improved blood circulation [6] and enhanced proliferation and differentiation rates of satellite cells [7]. Last but not least improved movement of tendon and fascia gliding against the surrounding tissues by stimulating lubricin expression by ESWs [8] may significantly contribute to myofascial trigger points release by ESWT.
Much less is known about the molecular and cellular mechanisms of neuroreflectory hyperbaric CO2 cryotherapy (NHCC) on the musculoskeletal system. One potential reason is that this therapy may have been considered just another way to cool down tissue somewhat faster than using ice bags or cold packs. However, recent research has indicated that NHCC primarily and mainly acts on and via the nervous system.
- Mourot et al. (2007) [9] exposed one hand of healthy people to NHCC and observed a rapid change in skin temperature (from 32.5° ± 0.5°C to 7.3° ± 0.8°C within 90 s) at the treated side, with a statistically significant reduction of the skin temperature at the contralateral side (to 30.5°± 0.6°C). When the authors did the same with an ice bag, the skin temperature changed from 32.5° ± 0.6° C to 13.9° ± 0.7° C at the treated side within 15 min, without significant change at the untreated side. This phenomenon can only be explained by a reaction of the peripheral and central nervous system. Mourot et al. (2007) [9] concluded that NHCC caused a systemic skin vasoconstriction response, whereas the vascular responses triggered by ice bag cooling appeared limited and localized to the cooled area.
- Demoulin et al. (2012) [10] reproduced the findings by Mourot et al. (2007) [9] that NHCC can result in faster and more profound reduction of skin temperature than application of a cold pack.
- Very recently, Kang et al. (2018) [11] divided a total of n=30 patients who had undergone shoulder rotator cuff reconstruction surgery into three groups (n=10 each), and treated them as follows: Group 1: continuous passive motion (CPM) and icing; Group 2: CPM and NHCC; and Group 3: CPM and microcurrent therapy. Each therapy was applied six days for two weeks. Pain, muscle tone near the shoulder area and levels of C-reactive protein (CRP) were tested before and after the interventions. Only the patients in Group 2 showed a statistically significant reduction in CRP levels (from 0.47 ± 0.03 mg / dl before the interventions to 0.28 ± 0.06 mg / dl after the interventions). Differences in shoulder muscle tone appeared only in the supraspinatus muscle, with Group 2 showing the biggest change. Kang et al. (2018) [11] concluded that NHCC may help to stabilize inflammation as well as to reduce pain and muscle tension when applied in patients following rotator cuff reconstruction.
These data indicate that NHCC must not be confused with ice bag / cold pack therapy, and the effects of NHCC are not restricted to the local area of treatment. Particularly the findings by Kang et al. (2018) [11] of significant anti-inflammatory effects of NHCC in postoperative therapy after surgical treatment of the rotator cuff suggest that the true potential of this therapy has so far been underestimated and not properly applied.
Summary
In summary, these case reports indicate that combination of rESWT and NHCC is safe and effective in treatment of painful conditions of the musculoskeletal system of athletes based on misuse and overloading. Future clinical trials are necessary to demonstrate the full potential of this novel combination therapy, and to clearly differentiate NHCC from other therapies with the aim of cooling the skin and underlying tissue (including ice bags, cold packs, air cooling devices, etc.).
You can request a detailed list of references under info@thesportgroup.de
Conflict of interest statement: RT has served as paid consultant for Axxana GmbH (Cologne, Germany), the distributor of the Cryolight device. CS has received an unrestricted research grant from Electro Medical Systems S.A. (Nyon, Switzerland), the manufacturer of the Swiss DolorClast device, at LMU Munich, and free loan of a Swiss DolorClast device from Electro Medical Systems GmbH (Munich, Germany) and a Cryolight device from Axxana GmbH for research purposes
at LMU Munich (maintenance and servicing paid by LMU Munich). CS served as paid consultant for Electro Medical Systems until the end of 2017. No other conflicts of interest related to the content presented in this article are reported.
Autoren
leitet die Praxis für Orthopädie und Sportmedizin am Brühl in Leipzig, ist Teamarzt des Handball Bundesligisten SC DHfK Leipzig und wiss. Beirat der sportärztezeitung.
ist Inhaber des Lehrstuhls II der Anatomischen Anstalt der Ludwig-Maximilians Universität München und wissenschaftlicher Beirat der sportärztezeitung.



