Hyaluronan (HA) has been used successfully in osteoarthritis treatment for over 30 years. Since a few years Platelet-rich Plasma (PRP) is applied in modified preparations and various indications e.g. in the treatment of gonarthrosis and progressive cartilage damage. Pain and loss of function are the main clinical symptoms that lead to treatment [13].
HA as well as PRP therapeutic applications show a positive effect regarding effectiveness, pain reduction and improvement of mobility over a period of up to 12 months, whereas HA levels off to a steady state of its positive effects after the first 6 months [12]. Although knee-replacement surgery provides an effective solution for severe knee osteoarthritis (OA), for younger and middle-aged patients with earlier stages of Arthritis, conservative nonsurgical interventions have been proposed [6, 24, 9]. Thus, the International Osteoarthritis Society recommends non-surgical treatment as a first line therapy [33]. Conservative interventions include analgesics, non-steroid and steroid anti-inflammatory drugs, corticosteroid and HA injections. More recently PRP, as a biological therapy, has become an intriguing treatment option to improve the status of the joint [5, 11, 10, 28, 29]. This raises the question whether the effects of HA and PRP is potentiated when a combination therapie of both is used and therefore leads to a possible therapy synergism.
Benefits of Hyaluronan
Currently, there is no evidence regarding the intraarticular therapy with PRP or HA, which advises one injection regimen superior to the other. The American Association of Orthopaedic Surgeons (AAOS) does not yet recommend HA in their guidelines for osteoarthritis, but current studies seem to update this recommendation [18, 23]. The Society of Orthopaedics and Orthopaedic Chirurgy (DGOOC) states in their updated S2k guideline of September 2020, “Intra-articular hyaluronic acid injection may be used in patients in whom the use of NSAIDs is contraindicated or in whom NSAIDs are not sufficiently effective” [27]. HA has viscoelastic as well as anti-inflammatory and cytoprotective properties, in addition to stimulating endogenous HA synthesis [26, 1, 14, 3, 4]. The rheological and mucoadhesive properties are particularly important for artificial joint fluids, as this elastic matter is able to successfully absorb mechanical energy and protect cartilage from damage, tear or abrasion [2]. This properties gain importance especially at high loads such as in competitive sports. The therapeutic effect and possible side effects of HA depend directly on the molecular weight of the biopolymer, its cross-linking, the dosage and its preperation [1, 26, 23, 17, 19] HA has its peak effect at about 2 months and lasts a total of about 6 months [10, 21]. (For further information please see sportärztezeitung 01/21 and Figure 1).
Benefits of PRP
The last few years, PRP has been used in various indications, including osteoarthritis and cartilage damage. PRP, as an autologous therapy, releases local growth factors, which in turn stimulate chondrocyte production. This enodgenous effect stimulates the extracellular matrix and could promote cartilage repair [11, 2]. Moreover, PRP has anti-inflammatory effects by increasing anti-inflammatory mediators, decreasing proinflammatory mediators and the expression of pro-inflammatory enzymes [22]. Further, PRP improves patient reported outcomes in terms of pain, functionality and stiffness, over a period of 6 – 12 months [10, 11, 30, 25, 16, 8, 12]. In some cases, the outcome after additional PRP injection is better than single HA injection therapy [10, 12, 29]. Especially in long-term outcome, PRP seems to be superior to HA solution [11, 15, 32]. Compared to saline, where significant functional impairment occurs after approximately 6 months, the improvement with PRP is consistently maintained over a 12-month period. This indicates that the improved outcome with PRP is not based on a placebo effect [10]. It was demonstrated in vitro that chondrocytes cultured with growth factors, which are also present in PRP, had higher proliferation rates than the control culture without growth factors [7]. Another topic that remains unclear is whether leukocyte-rich or leukocyte-poor PRP shows more benefits regarding functional and pain outcome parameters. However, based on current knowledge, it appears that leukocyte-rich PRP is more effective [5].
Combination Therapy – Potential for a therapy synergisms?
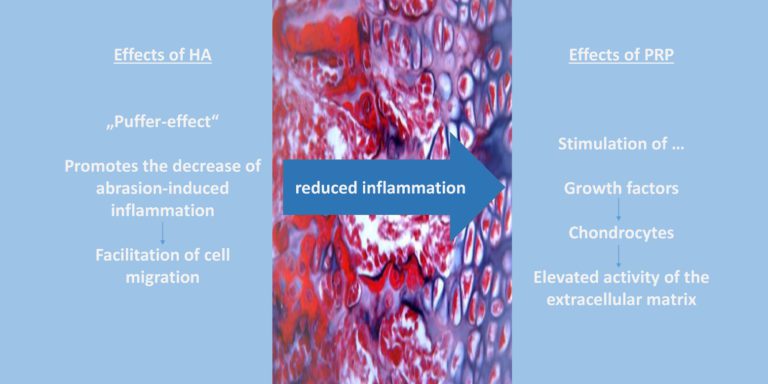
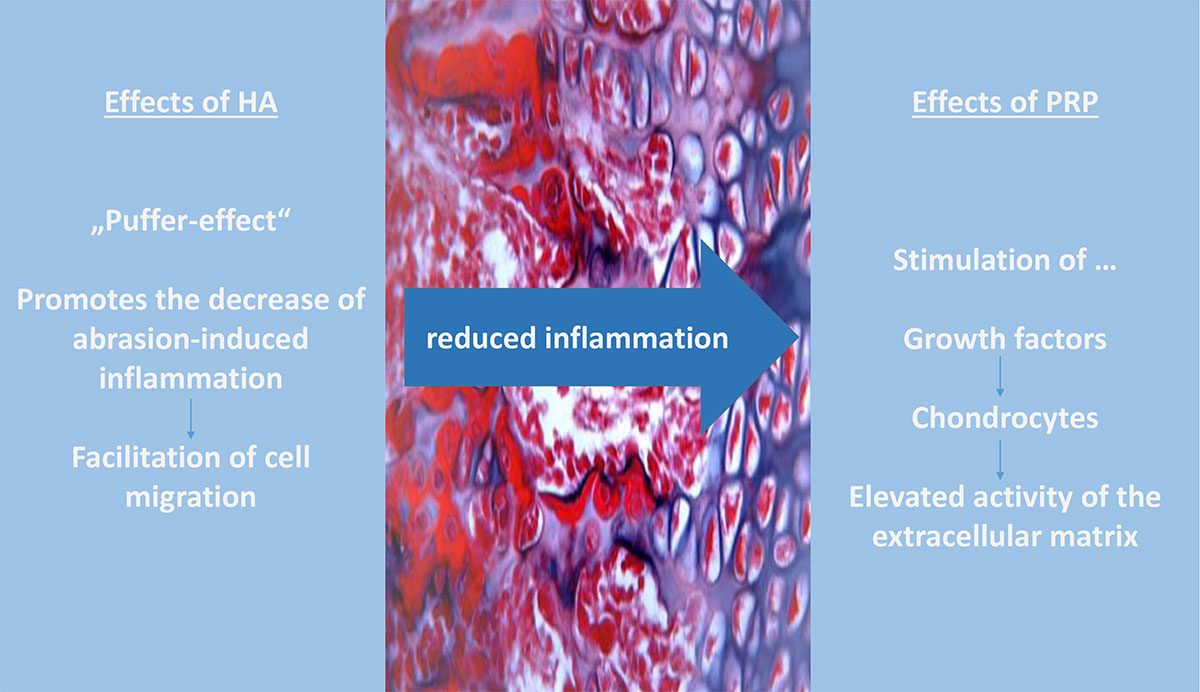
In case of a combination therapy, it can be differentiated between a direct combination therapy and an injection therapy with time intervals of e.g 48 h to 7 days between HA and PRP injection. With both therapy regimes a synergism would be conceivable. This synergism could be due to the anti-inflammatory and visco-elastic properties of action of HA and the additional chondrocyte stimulating and anti-inflammatory effect of PRP [33]. This synergistic effect has been partially demonstrated in experimental in-vitro cell and in-vivo animal experiments [30]. Increased chondrocyte proliferation and increased glycosaminoglycan concentration, decreased apoptosis and therefore less cartilage damage were observed [20]. Furthermore, it has been shown that synovial fibroblasts and tendon cells exhibited improved cell mobility in a PRP plus HA solution than in a PRP-only solution [33]. A current meta-analysis by Zhang et al. (2020) showed that a combined therapy of PRP and HA leads to significantly higher improvments in terms of pain perception and functionality compared to PRP or HA alone (after 6 months). Additionally, the PRP-HA group achieved greater improvement in WOMAC function score, WOMAC total score and Lequesne score at 12 months [32]. Gilat et al. (2021) concluded that combination therapy was superior to HA alone in terms of functionality and pain after 6 and 12 months [15]. However, long-term studies (> 12 months) are still lacking. Moreover, combination therapy only referred to the simultaneous administration of PRP and HA in one injection session. There is currently no evidence regarding a therapy success in terms of an injection regimen with a 48 h to 7 days interval between injections [33, 16]. The combination therapy of PRP and HA is not to be expected leading to an increased risk, compared to therapy with PRP or HA alone and shows comparable side effects [33]. No significantly increased rate of adverse events was demonstrated in either group [29, 28]. As described before, the properties of PRP would conceivably reduce joint inflammation and stimulate chondrogenesis [5]. Yet only Xu et al. have included examination (Doppler Imaging for Synovium and Cartilage, Synovial fluid for Inflammatory parameters) regarding these parameters [30]. A comprehensive pathophysiological concept does not yet exist, but a common therapeutic effect via the extracellular matrix is conceivable. This is activated by both the hydrophilic properties of HA and platelet growth factors. Future studies should investigate possible effects on various synovial parameters and the extent of cartilage damage on MRI, because evidence is lacking regarding objective parameters.
Limitations
One of the most frequently mentioned limiations is the heterogeneity in the preparation and injection of PRP, but also in the kind of injection of HA [11, 10, 5, 12, 8]. Too short follow-ups and lacking study designs were also criticized [33, 10, 25, 22]. Additionally, there were concerns about patient selection and heterogeneity among patients. The inclusion and exclusion criteria were not evaluated in detail and patients who had knee pain but no knee osteoarthritis were enrolled [8, 10, 33]. Some authors also missed post-injection radiographic or MRI data collected at follow-up [22, 5, 21].
Conclusion
The current study designs appear to be very heterogeneous. Standardized designs with more homogenous groups will be needed in order to develop evidence-based therapy [12, 31, 10, 5, 20]. Based on the literature PRP seems to be superior to HA alone in terms of pain and functionality in mid- and long-term follow-up [12, 31, 22, 5, 21]. However, recent studies show that the combination therapy of HA and PRP shows promising results (VAS, WOMAC function and total, Lequesne Index) after 12 months than PRP alone [33]. It can be concluded that, at the present time, further high quality RCTs using a standardized method of preparation and injection are needed to establish an evidence-based therapy. As mentioned above, additional examinations of the synovia, as well as cartilage imaging should be included in the study design in order to make solid statements about the influence on inflammation and cartilage repair to refer to more objective parameters. Additionally this would lead to a more valid statement about the influence of the injection therapy on inflammation and cartilage repair.
References
[1] Roy D Altman, Asheesh Bedi, Jon Karlsson, Parag Sancheti, and Emil Schemitsch. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. The American journal of sports medicine, 44(8):2158 – 2165, 2016.
[2] Isabel Andia and Michele Abate. Knee osteoarthritis: hyaluronic acid, platelet-rich plasma or both in association? Expert opinion on biological therapy, 14(5):635–649, 2014.
[3] Jeremie M Axe, Lynn Snyder-Mackler, and Michael J Axe. The role of viscosupple-mentation. Sports medicine and arthroscopy review, 21(1):18–22, 2013.
[4] Ilker S Bayer. Hyaluronic acid and controlled release: A review. Molecules, 25(11): 2649, 2020.
[5] John W Belk, Matthew J Kraeutler, Darby A Houck, Jesse A Goodrich, Jason L Dragoo, and Eric C McCarty. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. The American journal of sports medicine, 49(1):249–260, 2021.
[6] Johannes WJ Bijlsma, Francis Berenbaum, and Floris PJG Lafeber. Osteoarthritis: an update with relevance for clinical practice. The Lancet, 377(9783):2115–2126, 2011.
[7] Anita Brandl, Peter Angele, Christina Roll, Lucas Prantl, Richard Kujat, and Bernd Kinner. Influence of the growth factors pdgf-bb, tgf-β1 and bfgf on the replica-tive aging of human articular chondrocytes during in vitro expansion. Journal of Orthopaedic Research, 28(3):354–360, 2010.
[8] Kirk A Campbell, Bryan M Saltzman, Randy Mascarenhas, M Michael Khair, Nikhil N Verma, Bernard R Bach Jr, and Brian J Cole. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other ther-apies in the treatment of knee osteoarthritis? a systematic review of overlapping meta-analyses. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 31(11): 2213–2221, 2015.
[9] Andrew J Carr, Otto Robertsson, Stephen Graves, Andrew J Price, Nigel K Arden, Andrew Judge, and David J Beard. Knee replacement. The Lancet, 379(9823): 1331–1340, 2012.
[10] Ke-Vin Chang, Chen-Yu Hung, Fanny Aliwarga, Tyng-Guey Wang, Der-Sheng Han, and Wen-Shiang Chen.Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Archives of physical medicine and rehabilitation, 95(3):562–575,
[11] Zehan Chen, Chang Wang, Shishun Zhao Di You, Zhe Zhu, and Meng Xu. Platelet- rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta- analysis. Medicine, 99(11), 2020.
[12] Wen-Li Dai, Ai-Guo Zhou, Hua Zhang, and Jian Zhang. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized con- trolled trials. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 33(3): 659–670, 2017.
[13] Paul A Dieppe and L Stefan Lohmander. Pathogenesis and management of pain in osteoarthritis. The Lancet, 365(9463):965–973, 2005.
[14] I Gigis, E Fotiadis, A Nenopoulos, K Tsitas, and I Comparison of two dif- ferent molecular weight intra-articular injections of hyaluronic acid for the treatment of knee osteoarthritis. Hippokratia, 20(1):26, 2016.
[15] Ron Gilat, Eric D Haunschild, Derrick M Knapik, Aghogho Evuarherhe, Kevin C Parvaresh, and Brian J Cole. Hyaluronic acid and platelet-rich plasma for the man- agement of knee osteoarthritis.International Orthopaedics, 45(2):345–354, 2021.
[16] Yanhong Han, Hetao Huang, Jianke Pan, Jiongtong Lin, Lingfeng Zeng, Guihong Liang, Weiyi Yang, and Jun Liu. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Medicine, 20(7): 1418–1429, 2019.
[17] Yves Henrotin, Raghu Raman, Pascal Richette, Herv´e Bard, J¨org Jerosch, Thierry Conrozier, Xavier Chevalier,and Alberto Consensus statement on viscosup- plementation with hyaluronic acid for the management of osteoarthritis. In Seminars in arthritis and rheumatism, volume 45, pages 140–149. Elsevier, 2015.
[18] Charles Hummer, Felix Angst, Emil Schemitsch, Craig Whittington, Colleen Manitt, and Wilson Ngai. High molecular weight intraarticular hyaluronic acid for the treat- ment of knee osteoarthritis: network meta-analysis. Osteoarthritis and Cartilage, 27: S503–S504, 2019.
[19] David Jevsevar, Patrick Donnelly, Gregory A Brown, and Deborah S Vis- cosupplementationfor osteoarthritis of the knee: a systematic review of the evidence. JBJS, 97(24):2047–2060, 2015.
[20] Theofilos Karasavvidis, Trifon Totlis, Ron Gilat, and Brian J Platelet-rich plasma combined with hyaluronic acid improves pain and function compared with hyaluronic acid alone in knee osteoarthritis: A systematic review and meta-analysis. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 2020.
[21] Jos´e FSD Lana, Adam Weglein, Steve E Sampson, Eduardo F Vicente, Stephany CaresHuber, Clarissa V Souza, Mary A Ambach, Hunter Vincent, Aline Urban-Paffaro, Carolina MK Onodera, et al. Randomized controlled trial comparing hyaluronic acid, platelet-richplasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. Journal of stem cells & regenerative medicine, 12(2):69, 2016.
[22] Carlos J Meheux, Patrick C McCulloch, David M Lintner, Kevin E Varner, and Joshua D Harris. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 32(3):495–505, 2016.
[23] Mark Phillips, Christopher Vannabouathong, Tahira Devji, Rahil Patel, Zoya Gomes, Ashaka Patel, MykaelahDixon, and Mohit Differentiating factors of intra- articular injectables have a meaningful impact on knee osteoarthritis outcomes: a network meta-analysis. Knee Surgery, Sports Traumatology, Arthroscopy, pages 1–9, 2020.
[24] John Richmond, David Hunter, James Irrgang, ATC Morgan H Jones, Lynn Snyder- Mackler, MD Daniel Van Durme, Cheryl Rubin, Elizabeth G Matzkin, Robert G Marx, Bruce A Levy, et al. The treatment of osteoarthritis (oa) of the knee. J Bone Joint Surg Am, 92:990–3, 2010.
[25] Longxiang Shen, Ting Yuan, Shengbao Chen, Xuetao Xie, and Changqing Zhang. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. Journal of orthopaedic surgery and research, 12(1):1–12, 2017.
[26] Petr Snetkov, Kseniia Zakharova, Svetlana Morozkina, Roman Olekhnovich, and Mayya Uspenskaya. Hyaluronic acid: the influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers, 12 (8):1800, 2020.
[27] J St¨ov S2k-leitlinie gonarthrose 2018. Im Internet: https://www.awmf.org/uploads/txszleitlinien/033 − 004lS2kGonarthrose2018 − 011 − verlaengert.pdf.
[28] Jixiang Tan, Hong Chen, Lin Zhao, and Wei Huang. Platelet-rich plasma versus hyaluronic acid in the treatmentof knee osteoarthritis: a meta-analysis of 26 random- ized controlled trials. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 37(1):309–325, 2021.
[29] Siqi Tang, Xiaoshuai Wang, Peihui Wu, Peiqi Wu, Jiaming Yang, Zefeng Du, Shaoyu Liu, and Fuxin Wei.Platelet-rich plasma vs autologous blood vs corticosteroid in- jections in the treatment of lateral epicondylitis: A systematic review, pairwise and network meta-analysis of randomized controlled trials. Pm&r, 12(4):397–409, 2020.
[30] Zhe Xu, Zhixu He, Liping Shu, Xuanze Li, Minxian Ma, and Chuan Ye. Intra- articular platelet-rich plasma combined with hyaluronic acid injection for knee os- teoarthritis is superior to platelet-rich plasma or hyaluronic acid alone in inhibiting inflammation and improving pain and Arthroscopy: The Journal of Arthro- scopic & Related Surgery, 37(3):903–915, 2021.
[31] Heng Zhang, Ke Zhang, Xianlong Zhang, Zhenan Zhu, Shigui Yan, Tiansheng Sun, Ai Guo, John Jones, R Grant Steen, Bin Shan, et al. Comparison of two hyaluronic acid formulations for safety and efficacy (chase) study in knee osteoarthritis: a mul- ticenter, randomized, double-blind, 26-week non-inferiority trial comparingdurolane to artz. Arthritis research & therapy, 17(1):51, 2015.
[32] Hua-feng Zhang, Chen-guang Wang, Hui Li, Yu-ting Huang, and Zhi-jun Li. Intra- articular platelet-rich plasma versus hyaluronic acid in the treatment of knee os- teoarthritis: a meta-analysis. Drug design, development and therapy, 12:445, 2018.
[33] Jinlong Zhao, Hetao Huang, Guihong Liang, Ling-feng Zeng, Weiyi Yang, and Jun Liu. Effects and safety of the combination of platelet-rich plasma (prp) and hyaluronic acid (ha) in the treatment of kneeosteoarthritis: a systematic review and meta- analysis. BMC musculoskeletal disorders, 21(1):1–12,
Autoren
ist wissenschaftliche Koordinatorin am LANS Medicum. Sie hat sich zuvor an der Deutschen Sporthochschule Köln in Exercise Science and Coaching (M. Sc.) spezialisiert und war selbst im Schwimmleistungssport aktiv.
ist Facharzt für Orthopädie und Unfallchirurgie, Spezielle Unfallchirurgie und Sportmedizin. Er ist Gründer und Inhaber des LANS Medicum. Seine mannschaftsärztlichen Betreuungen umfassten u. a. das Handballteam des HSV sowie von 2011-2014 die Erstligafußballmannschaft des Hamburger SV. Heute betreut er mit seinem Team mehrere Fußball- und Hockeyteams sowie das Hamburger Ballett von John Neumeier. Außerdem ist Prof. Catalá-Lehnen als Professor für den Schwerpunkt Orthopädie an der Medical School Hamburg und am UKE in der Lehre für das Fach Knochenpathologie tätig.