In a disease burden study conducted by the Robert Koch Institute between October 2019 and March 2020 (BURDEN 2020) 5,009 adults were questioned about the frequency and intensity of back and neck pain. It was shown that 61.3 % of those questioned had suffered from back pain in the previous 12 months.
Lower back pain was about twice as common as upper back pain; 15.5 % of those questioned said they suffered from chronic back pain [1]. The causes of back pain are manifold. A distinction is drawn between specific and non-specific back pain. In the SK2 guidelines on specific lumbar or back pain, lumbar discogenic pain syndrome is mentioned alongside others as a separate entity in vertebral osteochondrosis, i.e. intervertebral disc-induced back pain [2].
Dehydration of the intervertebral discs
Intervertebral discs consist of a soft, inner gel-like core (nucleus pulposus) and an outer fibrous ring (annulus fibrosus). The ideal combination of a soft core and an outer ring acts as a cushion. In the course of life, however, the core dehydrates, i.e. loses water and “shrinks” (in much the same way as a grape becomes a raisin). The cause is a decline in chondrocytes, which produce chondroitin sulphate and proteoglycans. These molecules have a high water-binding capacity. The loss of these molecules leads to dehydration/drying out of the intervertebral disc, a process which is known as discopathy. The wear-and-tear process is readily visible in an MRI. The modified Pfirrmann grading system is generally used to assess the severity of discopathy. It defines 8 grades of severity [3]. It has been shown in various studies that discopathy is far more common in elite athletes than in non-athletes (75 % as opposed to 31 %) [4]. This seems to be especially the case in sports involving rotation of the upper body (tennis, baseball, golf) and in those that tend to cause hyperlordosis (butterfly and breaststroke in swimming) particularly if training begins in early childhood and adolescence [4 – 6].
Treatment options
Initial treatment for discogenic back pain, usually associated with reflex muscle tension, involves physiotherapy and pain medication, but may also include shock wave therapy, acupuncture etc. Both anti-inflammatory nutritional supplements (e.g. Omega 3 fatty acids, turmeric, ginger, chilli and Indian frankincense) and special products such as glucosamine, chondroitin and MSN are suitable supporting treatments. Micronutrients, which protect against oxidative stress, enzymes such as papain and bromelain and secondary plant metabolites (flavonoids and carotenoids) can support the healing process. To reduce loading on the intervertebral discs, delordosing back braces such as the Aspen Elite Active + are suitable specifically in the damaged lumbar area. This often results in an improvement in the symptoms. However, should the acute pain become chronic, the question then arises as to what other treatment should then be considered. Various destructive intradiscal procedures, such as intradiscal electrothermal therapy (IDET), nucleoplasty and percutaneous endoscopic laser discectomy, have been attempted. Success with these procedures has been limited in most cases, and there is also the added disadvantage that the destruction involves the removal of vital chondrocytes, which tends more to accelerate the wear-and-tear process [7]. It is for this reason that the use of biologics is increasingly being tested in clinical practice to achieve long-term alleviation of the inflammation and some degree of repair of the intervertebral discs. Biomolecular, cell-based and tissue-engineering strategies are followed, depending on the severity of the intervertebral disc damage [8]. With regard to biomolecular procedures, most of the clinical experience has been with the intradiscal application of platelet-rich plasma (PRP). Unlike other platelet-rich plasma products, the autologous conditioned plasma (ACP) used here has a low concentration of white blood cells (e.g. neutrophil granulocytes), which can hinder the healing process at high concentrations. ACP is a blood concentrate that contains natural concentrations of growth factors and cytokines and which is currently widely used in clinical settings for tissue regeneration and repair. It has the great potential of stimulating the cell proliferation and metabolic activity of intervertebral disc cells. Several studies have shown that an injection of platelet-rich plasma products into degenerated intervertebral discs can restore structural changes and improve matrix integrity. Furthermore, they can restore intervertebral disc height, initiate the healing of annular lacerations and have an anti-inflammatory effect by down-regulating inflammatory factors, thus helping reduce pain and improve bodily function [9, 10]. The platelet-rich plasma product ACP is collected by separating the patient’s blood in a centrifuge. Venous blood is withdrawn using the special ACP double-syringe system, which allows rapid sterile separation and plasma collection with a 2-3-fold increased platelet concentration within a safe, closed system.
Case report
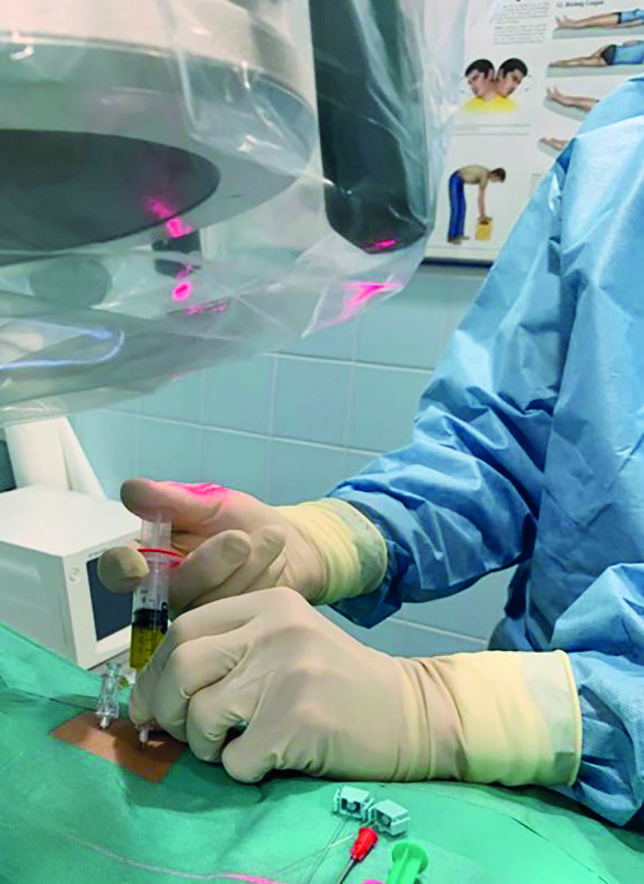
A 29-year-old recreational athlete (football) complained of back pain. The symptoms showed no signs of improvement despite conservative treatment with physiotherapy and pain medication. The patient complained of load-induced pain of 6 on the numerical rating scale (NRS) of 1–10. MRI was accordingly indicated. This showed grade IV discopathy at L4/5 and 5/S1 on the modified Pfirrmann scale [3]. There was also a high-intensity zone (HIZ) representing a fissure/tear in the posterior annulus at L4/5 (Fig. 1). High-intensity zones are a phenotype that shows as a hyperintense area of the intervertebral disc in a T2-weighted (T2W) MRI and which has been described as a potential imaging biomarker for identifying symptomatic intervertebral discs [11]. We consequently conducted provocative discography at L4/5 and 5/S1. Puncture of the intervertebral disc was performed in the trajectory/coaxial view. The patient lies in the prone position. The lumbar spine is delordosed with a roll. The entry point for the cannula is then marked. This involves positioning the image converter towards the centre of the intervertebral disc. For this, the base plate and cover plate are placed parallel to each other in the anterior-posterior (ap) beam view. The image converter is then swung to one side by approx. 45°, so that the annulus in the dorsal region is in the Parviz Kambin triangular safe zone (Fig. 2). We use coaxial cannulas size 23G/0.6, 100 mm or 21G/0.8, 120 mm for puncturing. These allow good guidance and their fineness significantly reduces the risk of nerve damage. Antibiotic prophylaxis is performed with Ampicillin 1 g iv 30 minutes before the procedure. The puncture is performed under sterile conditions. The position of the cannula is monitored in the ap beam and lateral beam view. Once the cannula is in the correct position in the centre of the intervertebral disc, contrast agent is applied via an injection pressure monitor to semiquantify the opening pressure. Depending on the pressure, approx. 1 – 1.5 mL contrast agent is applied intradiscally. This results in a further increase in pressure and, with a positive outcome, in typical memory pain, i.e. the pain the patient otherwise also feels during corresponding pain attacks (Fig. 3). The contrast agent should not drain out, i.e. the fibrous ring should be closed so that the ACP also remains within the disc.

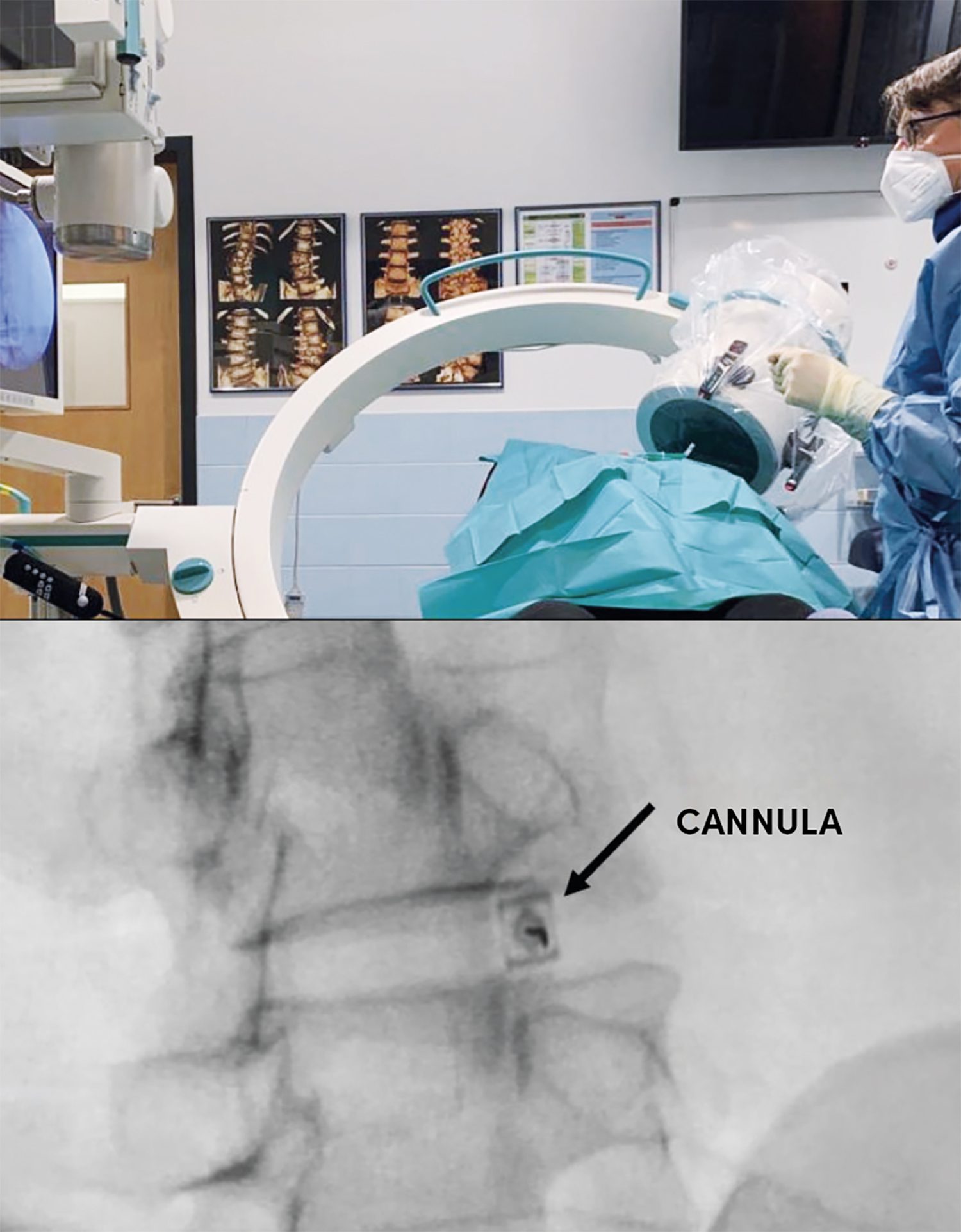
For this, the base plate and cover plate are placed parallel to each other in the anterior-posterior (ap) beam view. The image converter is then swung to one side by approx. 45o, so that the annulus in the dorsal region is in the Parviz Kambin triangular safe zone. With an ideal puncture, the cannula is visible as a dot (see arrow).

This manoeuvre was performed in our case study, thus allowing the intervertebral disc to be identified as the pain generator. The distribution of the contrast agent in the posterior section of the annulus is typical, as that is where the defects are localised (Fig. 3). As the patient had reacted positively to the provocative discography at both L4/5 and L5/S1, the application of ACP in both intervertebral discs was indicated. The puncture was performed for this purpose using the technique described above. Small amounts of contrast agent (0.2 – 0.3 mL) were applied to monitor the position of the cannula, followed by approx. 1.0 – 2.0 mL ACP up to a maximum of 20 PSI, depending on the pressure (Fig. 4). As expected, this provoked pain. Afterwards, the patient had to lie down on his back in the treatment room for approx. one hour to recover and was then able to leave the practice with an accompanying person. During this time, the ACP coagulates and becomes embedded in the intervertebral disc. We performed three injections at intervals of one week. As ACP therapy is an immunomodulatory therapy, improvements do not become manifest until at least six weeks after the start of treatment. Our patient presented three weeks after the last injection and reported a marked reduction in pain by approx. 50 % (NRS 3), with the pain often not occurring until after prolonged loading. He was also able to resume training. Physiotherapy was performed as an adjunct treatment to improve trunk stability.
Conclusion
Previous studies and our own experience show that the intradiscal injection of platelet-rich plasma products such as ACP is safe and feasible for the treatment of patients with back pain due to degenerative intervertebral discs (discopathy). This treatment seems to be particularly suitable for young and middle-aged adults when the discopathy is still moderate (up to grade V on the Pfirrmann scale). It has also been shown that a significant effect does not become manifest until at least 6 – 8 weeks afterwards and that further improvement is possible up to 6 months after the start of treatment.
Literature
[1] Robert Koch Institut. Journal of Health Monitoring | 2017/2 | Gesundheitsverhalten. 2017;
(2):1–120. Available from: https://www.rki.de/DE/Content/Gesundheitsmonitoring/Gesundheitsberichterstattung/GBEDownloadsJ/JoHM_2017_02_Gesundheitsverhalten.pdf?__blob=publicationFile
[2] S. Kroppenstedt, A. Halder. Spezifischer Kreuzschmerz [Internet]. 2017. Available from: https://www.awmf.org/leitlinien/detail/ll/033-051.html
[3] Griffith JF, Wang YXJ, Antonio GE, Choi KC, Yu A, Ahuja AT, et al. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine. 2007;32(24).
[4] Hangai M, Kaneoka K, Hinotsu S, Shimizu K, Okubo Y, Miyakawa S, et al. Lumbar intervertebral disk degeneration in athletes. American Journal of Sports Medicine. 2009;37(1):149 – 55.
[5] Ong A, Anderson J, Roche J. A pilot study of the prevalence of lumbar disc degeneration in elite athletes with lower back pain at the Sydney 2000 Olympic Games. British Journal of Sports Medicine. 2003;37(3):263 – 6.
[6] Fiani B, Jarrah R, Wong A, Alamah A, Runnels J. Repetitive Traumatic Discopathy in the Modern-Era Tennis Player. Cureus. 2020;12(8).
[7] Singh K, Ledet E, Carl A. Intradiscal therapy: a review of current treatment modalities. Spine [Internet]. 2005 Sep 1;30(17 Suppl):S20 – 6.
[8] Moriguchi Y, Alimi M, Khair T, Manolarakis G, Berlin C, Bonassar LJ, et al. Biological Treatment Approaches for Degenerative Disk Disease: A Literature Review of in Vivo Animal and Clinical Data. Global Spine Journal. 2016;6(5):497 – 518.
[9] Hirase T, Jack II RA, Sochacki KR, Harris JD, Weiner BK. Systemic Review: Is an Intradiscal Injection of Platelet-Rich Plasma for Lumbar Disc Degeneration Effective? Cureus. 2020;
12(6):6 – 13.
[10] Akeda K, Ohishi K, Masuda K, Bae WC, Takegami N. Intradiscal Injection of Autologous Platelet-Rich Plasma Releasate to Treat Discogenic Low Back Pain : A Preliminary Clinical Trial. 2017;11(3):380–9.
[11] Teraguchi M, Cheung JPY, Karppinen J, Bow C, Hashizume H, Luk KDK, et al. Lumbar high-intensity zones on MRI: imaging biomarkers for severe, prolonged low back pain and sciatica
Autoren
ist Neurochirurg und Sportmediziner und auf minimalinvasive, endoskopische und regenerative Behandlung von Rückenschmerzen spezialisiert. Er ist Ausbilder in der spinalen Endoskopie und Hochschullehrer an der Charité Universitätsmedizin Berlin. Wissenschaftlich ist er auf dem Gebiet der Bandscheibenregeneration klinisch und experimentell tätig.