In the last issues of the sportärztezeitung, but also online on our new sports medicine portal www.sportaerztezeitung.com, we focused on the topic of vitamins, particularly on vitamin D deficiency and the positive effects of vitamin D combined with vitamin K. The results collated to date with additional current information, including also vitamin C and the immune system, can be found at www.sportaerztezeitung.com/category/rubriken/ernaehrung/
We are delighted that our long-term partner and scientific advisor to the sportaerztezeitung, Klaus Pöttgen, has now picked up this thread, giving in this issue an interesting insight into current research and discussing further its importance for athlete care.
The important role played by vitamin D in bone metabolism and thus in sport has been studied in detail in recent years. The importance of an adequate supply of vitamin D in sports medicine, however, lies not only in regeneration following bone injury but also for many years in prevention and the positive effect on the immune system.
As presented in the article by Prof. Ghanaati, Dr Volz et al. in the 04/2020 issue of the sportärztezeitung (pp. 96-98), the following functions also play a role:
- anti-inflammatory effect via cytokines
- expression of proteins as transcription factors via vitamin D receptors on body cells (muscles)
- immunomodulation
- improved cardiopulmonary loading capacity.
Vitamin D and vitamin K
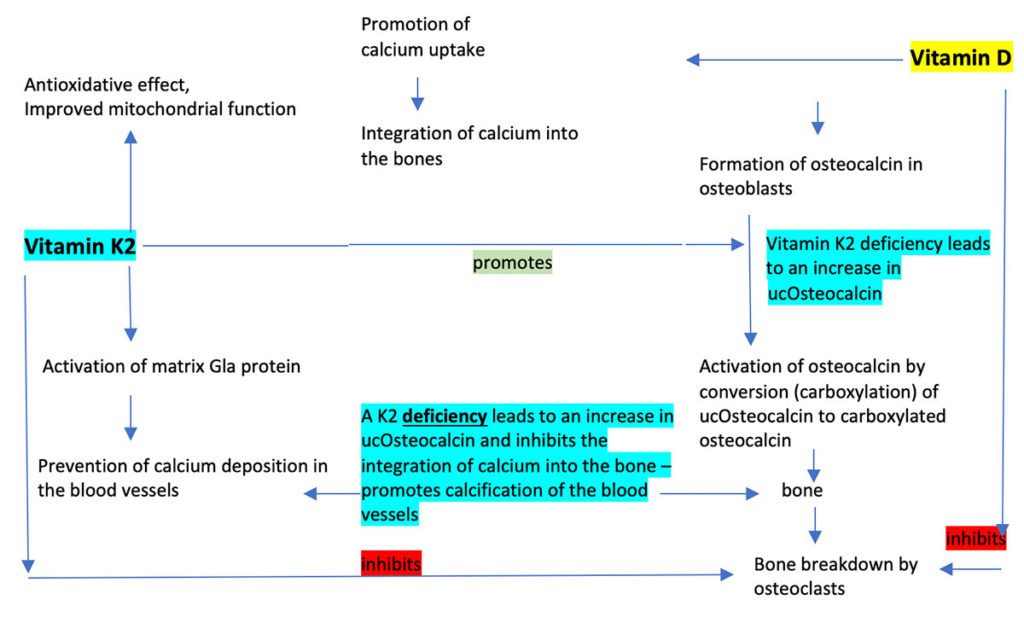
Without vitamin D, calcium ingested in food cannot be absorbed and used by the body. Vitamin D controls the synthesis of proteins that later require activation by vitamin K. The vitamin K group is named for its indirect effect on coagulation (after the Danish/German word “Koagulation”). Nowadays, a distinction is drawn between the different forms of the fat-soluble vitamin, primarily between K1 and K2 with its subforms (menaquinone – MK).
Caption: There is a traditional food in Japan called natto, which is made from soybeans that have been fermented with Bacillus subtilis and which contains high amounts of MK-7.
Vitamin K forms – supplied in the nutrition and by synthesis
Vitamin K1 (phylloquinone) is synthesised by plants and algae. 70-90% of this vitamin is ingested in green leafy vegetables such as kale, Brussels sprouts, broccoli and spinach and smaller amounts in soya oil, rapeseed oil, liver and eggs. Vitamin K2 (menaquinone) is both produced in small amounts in humans by the enzymatic conversion of K1 and ingested in its subforms in fermented products such as yoghurt and cheese, is formed by bacteria and occurs typically in amounts of less than 25% [1,2]. Following a review by the European regulatory body, the EFSA, vitamin K2 was approved for use in European food and nutrition supplements in 2009. In terms of prevention and treatment, menaquinone-4 (MK-4) and menaquinone-7 (MK-7) are the two most important vitamins in the vitamin K group [9,10]. There is a traditional food in Japan called natto, which is made from soybeans that have been fermented with Bacillus subtilis and which contains high amounts of MK-7 [3]. 10-50% of our daily K2 requirement is met by specific gut bacteria generally responsible for producing K2 [5,6] and requires a healthy individual gut microbiome for vitamin K-dependent physiological processes. MK-4 is the only menaquinone that cannot be formed by bacteria in the gut microbiome and is only produced in individual organs from plant-based K1 (phylloquinone) [4]. As there has been a marked deterioration in gut health and fewer fermented foods are being consumed, studies doubt that the amount of K2 produced by the body is sufficient to meet our daily K2 requirement and assume that vitamin K deficiency is widespread in western countries [5-8].
Metabolism – carboxylation
Cardiovascular system
Matrix Gla protein (MGP) and osteocalcin are the two extrahepatic vitamin K-dependent proteins that have been best studied [11]. Whereas osteocalcin promotes the integration of calcium into the bone matrix and thus supports bone metabolism, vitamin K-dependent MGP helps prevent calcification of the blood vessels, the development of inflammatory atherosclerosis with focal plaque formation and age-related wear and tear of the arteries. It thus protects the blood vessels against calcium overload [12,13]. Vitamin K2 activates MGP by carboxylation. It is only in this carboxylated state that it binds and transports free calcium in the blood [14,15]. In vitamin K2 deficiency, the calcium-binding proteins are undercarboxylated and thus inactive. In the 2004 Rotterdam heart study of 4,807 subjects over a ten-year period, it was shown that people who consumed foods naturally high in vitamin K2 (min. 32 µg daily) had markedly fewer calcium deposits in the arteries. Vitamin K2 reduced the risk of developing blood vessel calcification and of dying of cardiovascular disease by 50% [16]. Increased vitamin K2 intake lowered the mortality risk in elderly patients by 25% [18]. This was also shown in other studies to be the case for vitamin K2 alone but not for vitamin K1, some of which is converted to K2 [17]. This relation seems to be confirmed by increased blood vessel calcification under treatment with vitamin K antagonists [19]. In another study, rats were administered a vitamin K antagonist to induce calcification of the arteries. If rats were then given food containing vitamin K2, this reduced the calcium content of the arteries in the rats by 50%. Thus, vitamin K2 not only prevented calcification but even reversed it [20]. Vitamin K2 is an isoprenoid quinone like CoQ10, has structural and functional similarities with coenzyme Q10 and acts as an electron carrier in certain species of bacteria. Vitamin K2 was therefore ascribed an important function as an electron transporter in the mitochondria of eukaryotic cells and, as regards the mitochondria, was thought to mimic the role of coenzyme Q, which supports mitochondrial ATP production in the respiratory chain [21]. In 2019, however, it was shown that vitamin K2 cannot replace coenzyme Q10 as an electron carrier in the mitochondrial respiratory chain of mammal cells (mice with a genetic defect). Further studies on this subject are therefore necessary [22].
Bone
Active vitamin D regulates transcription of osteocalcin and only functions when it has been carboxylated by vitamin K. Vitamin D and vitamin K therefore act in synergy in bone formation. Vitamin K, primarily MK-7, also likely inhibits the transcription factor NFkB and by so doing helps improve bone formation [24]. Inadequately carboxylated calcitonin has a lower affinity to calcium phosphate in the bone matrix. Only carboxylated osteocalcin (cOc) promotes mineralisation of the bone matrix, increases bone strength and reduces the risk of fracture. Patients with atrial fibrillation who were not treated with vitamin K antagonists to prevent blood clotting had a lower risk of osteoporosis [23]. Elderly women with osteoporotic hip fractures had markedly lower serum levels of vitamin K [25]. Low vitamin K intake and high levels of undercarboxylated osteocalcin (ucOc) are independent risk factors for hip fractures [27-29]. In Japan, pharmacological doses of MK-4 (10-90 mg/d) are routinely used to treat osteoporosis and in postmenopausal women over a period of one to three years demonstrate a positive effect on maintaining bone density and fracture risk, as shown in a meta-analysis of 6,759 subjects [30].
K2 deficiency – undercarboxylated osteocalcin (ucOc)
In vitamin K deficiency, the calcium-binding proteins are undercarboxylated and thus inactive. Blood samples of healthy subjects showed that although all coagulation proteins had been completely carboxylated by vitamin K, most of the study subjects had high levels of undercarboxylated y-carboxyglutamate acid proteins (osteocalcin, matrix Gla protein). Undercarboxylated osteocalcin (ucOc) and matrix Gla protein (ucMGP) are functional laboratory parameters for vitamin K deficiency and are associated with an increased risk of bone fractures and/or blood vessel complications. Based on the results of this study, it must be assumed that a large part of the population does not have an adequate supply of vitamin K2 [31].
Administration of vitamin K2
There is increasing evidence that the recommended daily vitamin K requirement given by professional nutrition societies for the γ carboxylation of the osteocalcin and matrix Gla proteins cannot be adequately met by a normal nutrition [32]. Both MK-4 and MK-7 are almost completely ingested from food and supplements. However, there is a big difference in the half-lives of the two forms in the body: MK-4 is eliminated after just a few hours, whereas MK-7 remains available in the blood for a full 72 hours. MK-7 thus demonstrates a far better and longer efficacy [33-35]. The body can only process the trans form of MK-7, a fact which is therefore particularly important when selecting a vitamin K2 product [48]. However, in 2018 it was shown that some products contained only a minimal amount of the effective trans form [49]. As daily supplementation with MK-7 can significantly affect anticoagulation, the authors advise against administration in patients under treatment with vitamin K antagonists [35,36]. Increased ucOsteocalcin also increases the risk of other chronic inflammatory diseases, such as type 2 diabetes. In a study in 2020, separate or combined supplementation with vitamins D3 and K2 significantly lowered glucose levels and the proportion of functional pancreatic beta cells. Administration of D3 + K2 induced a reduction in the uOC/cOC index [37]. Apart from its function in calcium-dependent metabolic pathways initiated by vitamin D, vitamin K also has its own anti-inflammatory effect by inhibiting the expression of pro-inflammatory cytokines. As an electron carrier, vitamin K protects against oxidative stress and thus helps inhibit inflammatory processes. Studies in both humans and animals have shown that vitamin K2 improves not only insulin sensitivity due to the involvement of the vitamin K-dependent protein osteocalcin but also regulation of adipokine levels, anti-inflammatory properties and lipid-lowering effects in type 2 diabetes [38,39].
Laboratory tests
Tests to determine the level of vitamin K2 in the blood are unsuitable for status analysis, as vitamin K2 is fat soluble, and the tests only show the blood level on the day of testing but not what is stored in tissue. It is an unstable molecule and pre-analysis is difficult, involving centrifugation and frozen transportation to the laboratory. Furthermore, levels fluctuate dramatically due to the short biological half-life of (MK-4 – 1 h, MK-7 approx. 72 h). Although vitamin K1 increases osteocalcin carboxylation, it is only MK-7 intake that results in a further increase in carboxylation levels. Vitamin K2 is an essential cofactor of osteocalcin carboxylation, i.e., the conversion of undercarboxylated “uc” osteocalcin to its carboxylated form. Undercarboxylated osteocalcin correlates with low bone density and increased fracture risk [40]. When the supply of vitamin K2 is poor and/or bioactivity is low, only a small proportion of the osteocalcin is carboxylated. This leads to an increase in ucOsteocalcin. Increased values indicate absolute and/or functional vitamin K2 deficiency. Various authors recommend determining the uOC/cOC or cOC/uOC index or an index with the total osteocalcin level (uOC/tOC) [41-43].
Sports
In cases of insulin resistance, Vitamin K2 improves mitochondrial function by improving respiratory capacity and by enhancing biogenesis and the enzyme activities of mitochondrial complexes by SIRT1 (Sirtuin 1) signal transduction [44,45]. Sirtuin 1 is involved in the differentiation of muscle cells, metabolic switching to lipolysis and retardation of apoptosis. An in vitro study in 2018 showed that vitamin K2 (MK-4) has a positive effect on the migration and proliferation of muscle – two important early steps in myogenesis [46]. If improved cardiac function under K2 administration had hitherto only been demonstrated in ill patients, this was also successfully demonstrated in healthy athletes in 2017. An 8-week intake of vitamin K2 was associated in 26 aerobic trained male and female athletes with an increase in maximum heart rate capacity of 12% in the cycle ergometer test [47].
Football
Vitamin D: In preliminary tests on 28 players in a Bundesliga squad, the mean vitamin D level in summer was 47.45 µg/L (24.1 – 75). In 26 players in January, it was 32.5 µg/L (14.5 – 62.2), with 8 players more or less taking regular supplementation.
The table below of six of the players not taking supplementation is intended to illustrate how dramatically vitamin D levels can fall.
| Vitamin D level in µg/L | ||||||
| Player | 1 | 2 | 3 | 4 | 5 | 6 |
| Summer/August | 36.4 | 65.8 | 67.7 | 58 | 69 | 62 |
| Winter/January | 14.5 | 32.4 | 22.5 | 34.2 | 25 | 33 |
| Fall in µg/L | 21.9 | 33.4 | 45.2 | 23.8 | 44 | 29 |
Vitamin K2: 14 of the 28 players were shown to have increased ucOsteocalcin in the preliminary tests. This is an indication of vitamin K2 deficiency.
This could be followed up with nutrition counselling and a microbiome test. Vitamin D should be administered in combination with K2.
Conclusion
- In addition to its effect on bone health, vitamin K2 is also likely to play an important role in protecting arterial walls against calcification.
- Vitamin K2 in the trans form of MK-7 seems to be the best form of supplementation.
- Vitamin K should not be administered to patients under treatment with vitamin K antagonists.
- Tests to determine the level of vitamin K2 in the blood are unsuitable for status analysis.
- Increased values of ucOsteocalcin indicate absolute or functional vitamin K2 deficiency. (Some authors also recommend determining quotients of ucOsteocalcin/total calcitonin or ucOsteocalcin/cCalcitonin).
- In the above case, administration of vitamin D in combination with K2 is particularly to be recommended.
- Vitamin K2 has a positive effect on diabetes.
- There is evidence of improved mitochondrial function and increased aerobic performance.
lLterature
1
Conly JM, Stein K. The production of menaquinones (vitamin K-2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci 1992;16:307-343
2
Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr 1995;15:399-417
3
Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res 2012;56
4
Okano T, Shimomura Y, Yamane M et al. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem 2008;283:11270-11279
5
Conly JM, Stein KE. The absorption and bioactivity of bacterially synthesized menaquinones. Clin Invest Med.1993;16(1):45-57.
6.
Conly JM, Stein K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintainingcoagulation homeostasis. Prog Food Nutr Sci. 1992;16(4):307-343.
Walther B, Chollet M. Menaquinones, Bacteria, and Foods: Vitamin K2 in the Diet. In: Gordeladze JO, ed. Vitamin K2 – Vital for Health and Wellbeing. InTech; 2017. doi:10.5772/63712
Shea M, Booth S. Concepts and Controversies in Evaluating Vitamin K Status in Population-Based Studies. Nutrients. 2016;8(1):8. doi:10.3390/nu8010008
Dam H, Schonheyder F, Tage-Hansen E, Studies on the mode of action of vitamin K. Biochem J, 1936;30:1075–1079.
10.
McKee RW, Binkley SB, MacCorquodale DW, Thayer SA, Doisy EA. The isolation of vitamins K1 and K2. J Am Chem Soc, 1939; 61: 1295.
Elke Theuwissen, Ellen C Cranenburg, Marjo H Knapen, Elke J Magdeleyns, Kirsten J Teunissen, Leon J Schurgers, Egbert Smit, Cees Vermeer. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr. 2012 Nov 14;108(9):1652-7. doi: 10.1017/S0007114511007185. Epub 2012 Jan 31.
12
Braam LA, Hoeks AP, Brouns F et al. Beneficial effects of vitamin K on the elastic properties of the vessel wall in postmenopausal women: a follow-up study. Thromb Haemost. 2004; 91(2): 373-380.
13
Vermeer C, Shearer MJ, Zittermann A, et al. Beyond deficiency: potential benefits of increased intakes of vitamin K for bone and vascular health. Eur J Nutr, 2004; 43(6):1-11.
14
Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr 2009;29:89-110
15
Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature
1997;386:78-81
16.
Geleijnse u. a.: Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study In: J Nutr., 2004 Nov, 134(11), S. 3100–3105, PMID 15514282
17
Fusaro M, Noale M, Viola V et al. Vitamin K, Vertebral Fractures, Vascular Calcifications, and Mortality:
VItamin K Italian (VIKI) Dialysis Study. JBMR 2012;27:2271-2278
18
Beulens JW, Bots ML, Atsma F et al. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009;203:489-493
19
ChatrouML, Winckers K, Hackeng TM et al. Vascular calcification: the price to pay for anticoagulation therapy
with vitamin K-antagonists. Blood Rev 2012;26:155-166
Schurgers u. a.: Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats In: Blood, 2007 Apr 1, 109(7), S. 2823–2831, PMID 17138823
M. Vos et al. Vitamin K2 Is a Mitochondrial Electron Carrier That Rescues. Pink1 Deficiency. Science. 336, 2012, S. 1306–1310
22.
Cristina Cerqua, Alberto Casarin, Fabien Pierrel, Luis Vazquez Fonseca, Giampiero Viola, Leonardo Salviati, Eva Trevisson. Sci Rep. 2019 Apr 25;9(1):6553.doi: 10.1038/s41598-019-43014-y. Vitamin K2 cannot substitute Coenzyme Q 10 as electron carrier in the mitochondrial respiratory chain of mammalian cells.
Huei-Kai Huang, Peter Pin-Sung Liu, Jin-Yi Hsu, Shu-Man Lin, Carol Chiung-Hui Peng , Jen-Hung Wang, Jih-I Yeh, Ching-Hui Loh. Risk of Osteoporosis in Patients With Atrial Fibrillation Using Non-Vitamin K Antagonist Oral Anticoagulants or Warfarin. J Am Heart Assoc. 2020 Jan 21;9(2):e013845.doi: 10.1161/JAHA.119.013845. Epub 2020 Jan 10.
24.
Masayoshi Yamaguchi 1 , M Neale Weitzmann. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int J Mol Med. 2011 Jan;27(1):3-14.doi: 10.3892/ijmm.2010.562. Epub 2010 Nov 11.
S J Hodges , K Akesson, P Vergnaud, K Obrant, P D Delmas. Circulating levels of vitamins K1 and K2 decreased in elderly women with hip fracture. J Bone Miner Res. 1993 Oct;8(10):1241-5.doi: 10.1002/jbmr.5650081012.
26
Szulc P, Chapuy M-C, Meunier PJ et al. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest, 1993; 91(4): 1769-1774.
27
Feskanich D, Weber P, Willett WC et al. Vitamin K intake and hip fractures in women: a prospective study. Am J Clin Nutr, 1999; 69(1): 74-79.
28
Yamauchi M, Yamaguchi T, Nawata K et al. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr, 2010; 29(6): 761-765.
29
Price PA. Role of vitamin-K-dependent proteins in bone metabolism. Annu Rev Nutr, 1988; 8: 565-583.
Z-B Huang , S-L Wan, Y-J Lu, L Ning, C Liu, S-W Fan. Does vitamin K2 play a role in the prevention and treatment of osteoporosis for postmenopausal women: a meta-analysis of randomized controlled trials. Osteoporos Int. 2015 Mar;26(3):1175-86. doi: 10.1007/s00198-014-2989-6. Epub 2014 Dec 17.
Theuwissen E, Magdeleyns EJ, Braam LA, Vitamin K-status in healthy volunteers. Food Funct, 2014; 5(2):229-234.
32
Theuwissen E, Magdeleyns EJ, Braam LA, Vitamin K-status in healthy volunteers. Food Funct, 2014; 5(2):229-234.
33
Sato T, Schurgers LJ, Uenishi K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr J. 2012;11:93. doi:10.1186/1475-2891-11-93
34
Koitaya N, Ezaki J, Nishimuta M, et al. Effect of Low Dose Vitamin K2 (MK-4) Supplementation on Bio-Indices in Postmenopausal Japanese Women. Journal of Nutritional Science and Vitaminology. 2009;55(1):15-21. doi:10.3177/jnsv.55.15
35
Sato T, Schurgers LJ, Uenishi K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr J, 2012; 11:93. doi: 10.1186/1475-2891-11-93
36
Theuwissen E, Teunissen KJ, Spronk HM et al. Effect of low-dose supplements of menaquinone-7 (vitamin K2) on the stability of oral anticoagulant treatment: dose-response relationship in healthy volunteers. J Thromb Haemost, 2013; 11(6): 1085-1092.
37
J I Aguayo-Ruiz , T A García-Cobián, S Pascoe-González, S Sánchez-Enríquez, I M Llamas-Covarrubias, T García-Iglesias, A López-Quintero , M A Llamas-Covarrubias, J Trujillo-Quiroz, E A Rivera-Leon. Effect of supplementation with vitamins D3 and K2 on undercarboxylated osteocalcin and insulin serum levels in patients with type 2 diabetes mellitus: a randomized, double-blind, clinical trial. Diabetol Metab Syndr. 2020 Aug 18;12:73.doi: 10.1186/s13098-020-00580-w. eCollection 2020.
38
Prasenjit Manna, Jatin Kalita. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: A review. Nutrition. Jul-Aug 2016;32(7-8):732-9. doi: 10.1016/j.nut.2016.01.011. Epub 2016 Jan 25.
39
Yan Li, Jie Peng Chen, Lili Duan , Shuzhuang Li. Effect of vitamin K2 on type 2 diabetes mellitus: A review. Diabetes Res Clin Pract. 2018 Feb;136:39-51.doi: 10.1016/j.diabres.2017.11.020. Epub 2017 Dec 2.
40
Yoshio Suzuki, Asako Maruyama-Nagao, Keishoku Sakuraba, Sachio Kawai. Level of serum undercarboxylated osteocalcin correlates with bone quality assessed by calcaneal quantitative ultrasound sonometry in young Japanese females. Exp Ther Med. 2017 May;13(5):1937-1943. doi: 10.3892/etm.2017.4206. Epub 2017 Mar 9.
41
Bourron Olivier, Phan Franck. Vitamin K: a nutrient which plays a littleknown role in glucose metabolism. Curr Opin Clin Nutr Metab Care. 2019;22(2):174–81.
42
Razny U, Fedak D, Kiec-Wilk B, Goralska J, Gruca A, Zdzienicka A, Kiec- Klimczak M, Solnica B, Hubalewska-Dydejczyk A, Malczewska-Malec M. Carboxylated and undercarboxylated osteocalcin in metabolic complications of human obesity and prediabetes: osteocalcin in obese and prediabetic patients. Diabetes/Metab Res Rev. 2017;33(3):e2862.
43
Villafán-Bernal JR, Llamas-Covarrubias MA, Muñoz-Valle JF, Rivera-León EA, González-Hita ME, Bastidas-Ramírez BE, Gurrola-Díaz CM, Armendáriz- Borunda JS, Sánchez-Enríquez S. A cut-point value of uncarboxylated to carboxylated index is associated with glycemic status markers in type 2 diabetes. J Investig Med. 2014;62(1):33–6.
44
Xiangni Su , Wenchen Wang, Congwen Fang , Chunping Ni , Jian Zhou, Xiaohui Wang, Lei Zhang , Xiaona Xu , Rui Cao , Hongjuan Lang , Feng Wang. Vitamin K2 Alleviates Insulin Resistance in Skeletal Muscle by Improving Mitochondrial Function Via SIRT1 Signaling. Antioxid Redox Signal. 2021 Jan 10;34(2):99-117. doi: 10.1089/ars.2019.7908. Epub 2020 Aug 19.
45
Hao-Hao Zhang, Gui-Jun Qin, Xia-Lian Li, Ying-Hui Zhang, Pei-Jie Du, Peng-Yu Zhang, Yan-Yan Zhao, Jing Wu. SIRT1 overexpression in skeletal muscle in vivo induces increased insulin sensitivity and enhanced complex I but not complex II-V functions in individual subsarcolemmal and intermyofibrillar mitochondria. J Physiol Biochem. 2015 Jun;71(2):177-90. doi: 10.1007/s13105-015-0396-x. Epub 2015 Mar 18.
46
Sissel Beate Rønning, Mona Elisabeth Pedersen, Ragnhild Stenberg Berg, Bente Kirkhus , Rune Rødbotten. Vitamin K2 improves proliferation and migration of bovine skeletal muscle cells in vitro. PLoS One. 2018 Apr 4;13(4):e0195432. doi: 10.1371/journal.pone.0195432. eCollection 2018.
47
Brian K McFarlin, Andrea L Henning, Adam S Venable. Oral Consumption of Vitamin K2 for 8 Weeks Associated With Increased Maximal Cardiac Output During Exercise. Altern Ther Health Med. 2017 Jul;23(4):26-32.
48
Wu, S., Liu, S., Davis, C. H., Stafford, D. W., Kulman, J. D., & Pedersen, L. G. (2011). A hetero-dimer model for concerted action of vitamin K carboxylase and vitamin K reductase in vitamin K cycle. Journal of theoretical biology, 279(1), 143-149
49
Szterk A, Zmysłowski A, Bus K (2018) Identification of cis / trans isomers of menaquinone-7 in food as exemplified by dietary supplements. Food Chemistry 243:403–409 DOI: 10.1016/j.foodchem.2017.10.001
Autoren
ist leitender Arzt BAD Gesundheitsvorsorge und Sicherheitstechnik GmbH. Von 2011 bis 2016 und 2019 bis 2020 war er Teamarzt des SV Darmstadt 98 und von 2015–2022 Arzt im Nachwuchsleistungszentrum. Von 2022–2023 ergänzte er das medizinische Team des 1. FC Kaiserslautern in den Bereichen Ernährungsmedizin, Regeneration und Leistungsmedizin und als Mannschaftsarzt. Von 2002 bis 2014 war er medizinischer Leiter Ironman Germany und ist im wiss. Beirat der Deutschen Triathlon Union (DTU). Außerdem ist er wiss. Beirat der sportärztezeitung.




