Muscle, ligament and tendon injuries are associated with an inflammatory process due to tissue damage, which manifests itself in the five cardinal symptoms of inflammation: pain, swelling, redness, warmth and loss of function. Acute inflammation is nevertheless a physiologically important process with the aim of repairing and regenerating the damaged tissue and restoring homeostasis [1].
In contrast, chronic unresolved inflammation, even if only weakly expressed, is destructive and contributes to prominent diseases such as arthritis / arthrosis, asthma, Crohn’s disease, Parkinson’s disease and Alzheimer’s disease, as well as atherosclerosis, diabetes and cancer [2]. It is therefore not surprising that anti-inflammatory corticoids and non-steroidal anti-rheumatics (NSAR) such as ibuprofen, aspirin or diclofenac are among the most frequently taken medications. However, their therapeutic efficacy is often unsatisfactory, especially with long-term use, and the side effects, such as immunosuppression, renal dysfunction and gastric ulcers, are severe and often cause discontinuation of the medication [3].
Promoting the resolution of inflammation as a new therapeutic approach
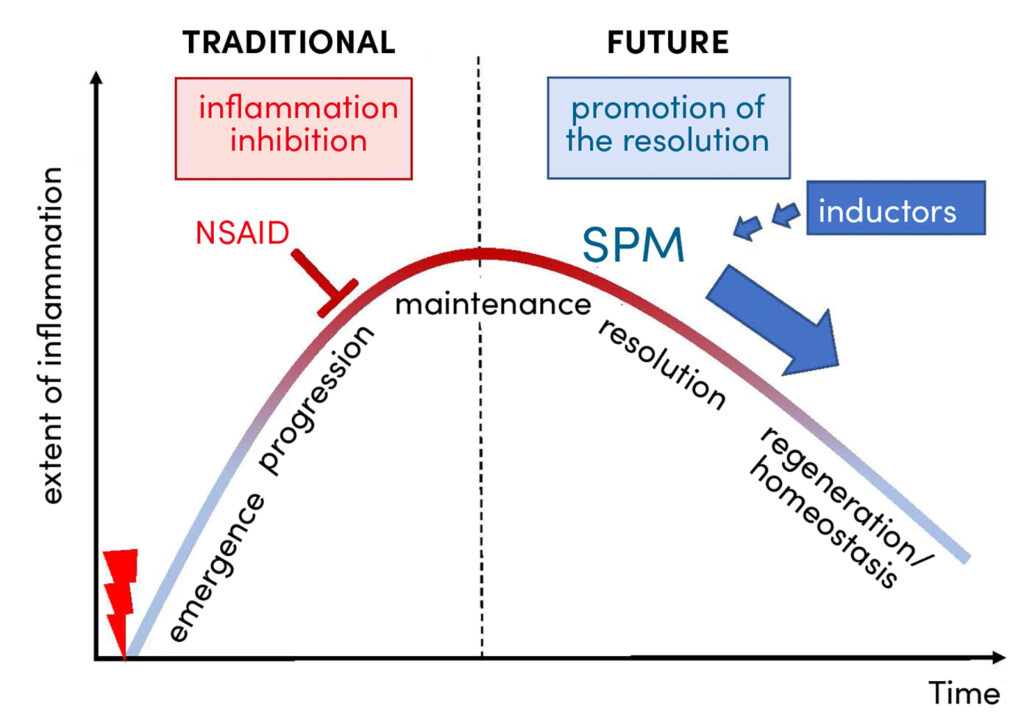
In order to develop alternative strategies for pharmacotherapy, basic research is endeavouring to gain a better understanding of the inflammatory process in its phases (i) development, (ii) progression, (iii) maintenance, (iv) inflammation resolution and (v) tissue repair and regeneration (Fig. 1). It has been recognised that, in contrast to conventional therapy with corticoids and NSAIDs, it is not the inhibition of inflammation but the promotion of inflammation resolution that could represent an attractive therapeutic option (Fig. 1) [4]. However, there are currently no approved drugs available for the latter. For a long time, it was thought that the resolution of inflammation was a passive process in which inflammatory messengers are eliminated. However, new findings from current research show that resolution is an actively controlled process in which messengers play a crucial role [5]. Inflammation resolution is therefore essential for a successful regeneration phase and thus important for the healing of tissue injuries. But how is this special resolution process regulated and which messenger substances and molecular mechanisms are involved?
Lipid mediators regulate all phases of inflammation
Lipid mediators are endogenous messengers that are formed primarily in immunocompetent cells and endothelial cells from polyunsaturated fatty acids. They are involved in all phases of inflammation, i.e. in the development, progression, resolution and tissue regeneration [5, 6]. While the omega-6 fatty acid arachidonic acid is converted by cyclooxygenases and 5-lipoxygenase into pro-inflammatory prostaglandins (PG) and leukotrienes (so-called eicosanoids), specialised pro-resolving mediators (SPM) can be formed from omega-3 fatty acids such as eicosapentaenoic acid and docosahexaenoic acid by means of various lipoxygenases (Fig. 2). These SPM comprise a large family (currently 47 known) of inflammation-resolving lipid mediators, which are subdivided into lipoxins, resolvins, protectins and maresins and are mainly formed by 12 / 15-lipoxygenases. Interestingly, eicosanoids, which are massively produced right at the beginning of the inflammation, and SPM, which are formed later in the process (Fig. 1), usually have opposing effects in the inflammatory process:
Eicosanoids
- induce inflammation, pain, fever and bronchoconstriction
- increase vascular permeability
- stimulate excessive phagocyte migration and activation
- induce the release of oxygen radicals, proteases and cytokines
SPMs, on the other hand
- promote the resolution of inflammation and reduce pain
- inhibit massive phagocyte infiltration and the release of pro-inflammatory cytokines
- stimulate phagocytosis and efferocytosis of apoptotic cells and cell debris
- improve wound healing and tissue regeneration and are organ-protective
Despite their pro-inflammatory character, PG also have beneficial and important properties for homeostasis. These include, among other things, the regulation of platelet aggregation, broncho- and vasodilation, gastric protection, kidney and uterine function. Of particular interest are the contrasting effects of PGE2 in the inflammatory process.
Depending on (i) the expression pattern of the four PGE2 receptors in the target cells, (ii) the amount of PGE2, and (iii) the temporal course of the inflammation, PGE2 has a pro-inflammatory effect in high amounts in the early inflammatory phase, but in the late phase, at low concentrations, it has an anti-inflammatory effect and promotes SPM formation and resolution / tissue regeneration [7]; the homeostatic PGE2 effects (gastric protection, kidney function) are also mediated by low concentrations [8].
Traditional and new strategies in inflammation therapy
Traditional anti-inflammatory therapy with NSAIDs is based on the blockade of COX-1/2 and thus on the suppression of the entire PG formation (Fig. 2). In view of the many different effects of PGE2, the question arises: How does the inhibition of PGE2 biosynthesis by NSAIDs affect resolution/regeneration? Experimental and clinical studies show that NSAIDs such as ibuprofen, indomethacin or celecoxib actually inhibit the resolution of inflammation by, for example, promoting leukotriene formation and simultaneously inhibiting SPM production [3, 9 – 11]. Thus, NSAIDs can have a negative effect on the healing of a tissue injury. But what alternatives to pharmacological inflammation intervention are there that do not inhibit resolution but instead specifically promote the resolution of inflammation? One approach currently being pursued is the direct application of SPM [12]. However, SPM are relatively unstable, their production in sufficient quantities is complex and expensive, and their targeted application is complicated. Another strategy to promote resolution through SPM consists of the supplementation of omega-3 fatty acids (DHA and EPA) and/or SPM precursors (18-HETE, 17-HDHA, 14-HDHA) to increase endogenous SPM formation. However, according to several clinical studies, this is not always successful; for example, SPM plasma levels are often unchanged after DHA/EPA supplementation [13].
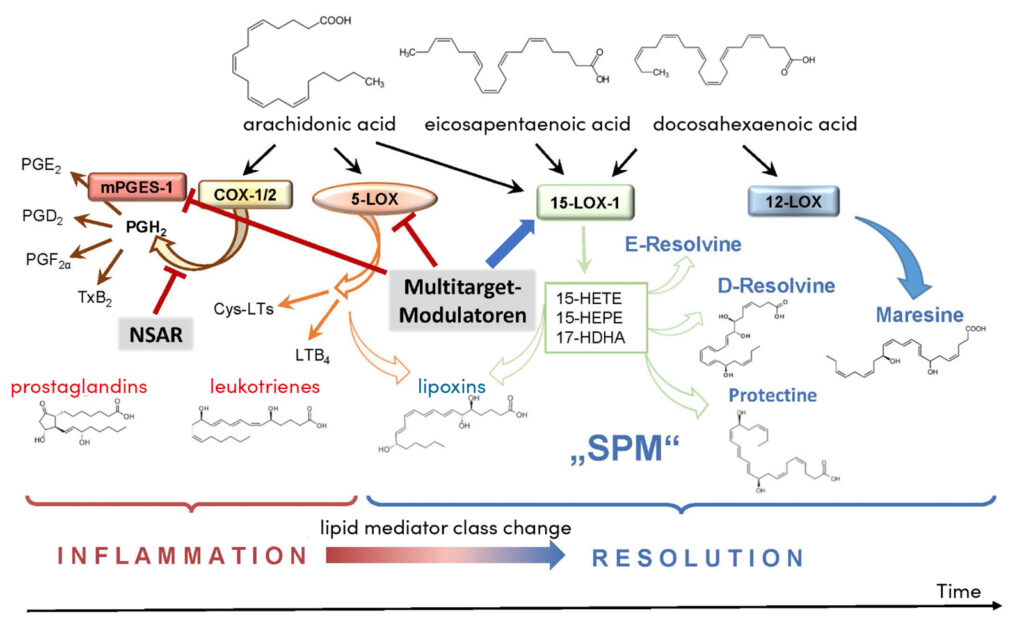
New strategies are pursuing the manipulation of endogenous SPM formation by modulating the biosynthetic enzymes. The aim here is to use special active ingredients to favourably influence the shift in the formation of eicosanoids towards SPM (so-called ‘lipid mediator class switch’ [7]), which occurs during resolution (Fig. 2). This could be used to specifically accelerate the healing of an inflammatory disease without causing the side effects typical of NSAIDs [14]. Some substances have already been identified for this purpose. Interestingly, natural products and phytopharmaceuticals are particularly active, which has been demonstrated by elucidating the molecular mechanisms of action and by experimental studies. In contrast to the COX-blocking NSAIDs, the pharmacological principle consists of specifically inhibiting those enzymes in the lipid mediator metabolism that produce high amounts of eicosanoids in the acute phase in inflammatory cells, without affecting anti-inflammatory PG (esp. PGD2 and PGE2) in the late phase and also activating the SPM-producing enzymes [14]. Specifically, these are substances that ideally combine three molecular mechanisms of action: inhibition of (i) microsomal PGE2 synthase-1 (mPGES-1; PGE2) and (ii) 5-lipoxygenase (leukotrienes) and (iii) stimulation of 12 / 15-lipoxygenases (SPM) (Fig. 2). Since these enzymes all have fatty acids or oxygenated fatty acids as substrates and are partly regulated allosterically by these molecules, so-called fatty acid mimetics are suitable as ‘multitarget modulators’ [8].
Natural products to promote the resolution of inflammation and regeneration
Numerous clinical studies demonstrate the efficacy of natural product-based drugs in inflammatory diseases, as shown, for example, by a meta-analysis of six controlled studies with patients with knee osteoarthritis: an enzyme-flavonoid combination preparation (Wobenzym) was comparable to diclofenac in terms of efficacy, but with better tolerability and fewer side effects [15].
The most well-known natural substances for the dissolution of inflammation are boswellic acids (pentacyclic triterpene acids and thus fatty acid mimetics) from the resin of frankincense trees, which have been identified as multitarget modulators for promoting the ‘lipid mediator class change’ [16]. Frankincense resin extracts have been used in folk medicine for the treatment of inflammatory diseases for many years, and more than a dozen clinical studies have demonstrated their efficacy in osteoarthritis, rheumatoid arthritis, chronic inflammatory bowel disease and asthma [16]. For more than 30 years, these anti-inflammatory effects have been explained by the modulation of lipid mediator biosynthesis, although the exact molecular mechanisms have only recently been elucidated. It has recently been shown that the lead compound acetyl-keto-boswellic acid (AKBA) is able to programmes 5-lipoxygenase in immune cells relevant to inflammation by allosteric binding in such a way that the formation of leukotrienes is inhibited and the enzyme takes on the function of a 12 / 15-lipoxygenase and thus produces increased levels of SPM [17]. SPM formation is further enhanced in immune cells by AKBA binding to a very specific site on 15-lipoxygenase-1, thereby directly activating this enzyme to produce SPM [18]. Results from experiments with mice in an inflammation model confirm this mode of action in living organisms as well and show an accelerated resolution of inflammation. In addition, classical boswellic acid acts as an inhibitor of mPGES-1 and inhibits, for example, excessive PGE2 formation and inflammation in mice in the peritonitis model [19]. This makes boswellic acids prototypes for modulating ‘lipid mediator class switching’ and models for other active ingredients that could be used to promote inflammation resolution. In fact, celastrol, a pentacyclic triterpenoid acid from the anti-inflammatory Wilfords three-winged fruit, shows similar molecular interactions with 5-lipoxygenase and 15-lipoxygenase-1 as AKBA. It thus suppresses the formation of leukotrienes and stimulates that of SPM [20]. But structurally different active ingredients such as chalcones from the Asian medicinal plant Melodorum fruticosum [21], a biflavinoid from the Cambodian dragon tree (Dracaena cambodiana) [22] or the custom-made synthetic substance BRP-201 [23] also promote ‘lipid mediator class switching’ in human immune cells and in the mouse peritonitis model by targeted multitarget modulation. Finally, the approved phytotherapeutic Traumeel® S, consisting of 14 mainly herbal ingredients, including arnica, witch hazel, echinacea, calendula and comfrey, was shown to promote resolution [24]. The various active ingredients intervene at several points in the inflammatory process and improved the ratio of eicosanoids to SPM in human cells in vitro and in the mouse peritonitis model in vivo.
Conclusion
In summary, the targeted modulation of multiple enzymes in lipid mediator metabolism by these active ingredients offers new options for the development of alternative strategies for the treatment of inflammation, with the aim of promoting the resolution of inflammation and regeneration. And all this without the side effects of previous anti-inflammatory drugs.
Bibliography
[1] M.L. Meizlish, R.A. Franklin, X. Zhou, R. Medzhitov, Tissue Homeostasis and Inflammation, Annu Rev Immunol 39 (2021) 557-581.
[2] I. Tabas, C.K. Glass, Anti-inflammatory therapy in chronic disease: challenges and opportunities, Science 339(6116) (2013) 166-72.
[3] K.D. Rainsford, Anti-inflammatory drugs in the 21st century, Subcell Biochem 42 (2007) 3-27.
[4] C.N. Serhan, B.D. Levy, Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators, The Journal of clinical investigation 128(7) (2018) 2657-2669.
[5] C.N. Serhan, Pro-resolving lipid mediators are leads for resolution physiology, Nature 510(7503) (2014) 92-101.
[6] P.C. Calder, Eicosanoids, Essays Biochem 64(3) (2020) 423-441.
[7] B.D. Levy, C.B. Clish, B. Schmidt, K. Gronert, C.N. Serhan, Lipid mediator class switching during acute inflammation: signals in resolution, Nat Immunol 2(7) (2001) 612-9.
[8] A. Koeberle, O. Werz, Natural products as inhibitors of prostaglandin E2 and pro-inflammatory 5-lipoxygenase-derived lipid mediator biosynthesis, Biotechnol Adv 36(6) (2018) 1709-1723.
[9] D.W. Gilroy, P.R. Colville-Nash, D. Willis, J. Chivers, M.J. Paul-Clark, D.A. Willoughby, Inducible cyclooxygenase may have anti-inflammatory properties, Nat Med 5(6) (1999) 698-701.
[10] J.F. Markworth, L. Vella, B.S. Lingard, D.L. Tull, T.W. Rupasinghe, A.J. Sinclair, K.R. Maddipati, D. Cameron-Smith, Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment, Am J Physiol Regul Integr Comp Physiol 305(11) (2013) R1281-96.
[11] M. Werner, P.M. Jordan, E. Romp, A. Czapka, Z. Rao, C. Kretzer, A. Koeberle, U. Garscha, S. Pace, H.E. Claesson, C.N. Serhan, O. Werz, J. Gerstmeier, Targeting biosynthetic networks of the proinflammatory and proresolving lipid metabolome, FASEB J (2019) fj201802509R.
[12] N. Chiang, C.N. Serhan, Specialized pro-resolving mediator network: an update on production and actions, Essays Biochem 64(3) (2020) 443-462.
[13] P.C. Calder, Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake, Biochimie 178 (2020) 105-123.
[14] N.C. Gilbert, M.E. Newcomer, O. Werz, Untangling the web of 5-lipoxygenase-derived products from a molecular and structural perspective: The battle between pro- and anti-inflammatory lipid mediators, Biochem Pharmacol 193 (2021) 114759.
[15] M. Abdel-Tawab, O. Werz, M. Schubert-Zsilavecz, Boswellia serrata: an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data, Clin Pharmacokinet 50(6) (2011) 349-69.
[16] N.C. Gilbert, J. Gerstmeier, E.E. Schexnaydre, F. Börner, U. Garscha, D.B. Neau, O. Werz, M.E. Newcomer, Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products, Nature chemical biology 16(7) (2020) 783-790.
[17] F. Borner, S. Pace, P.M. Jordan, J. Gerstmeier, M. Gomez, A. Rossi, N.C. Gilbert, M.E. Newcomer, O. Werz, Allosteric Activation of 15-Lipoxygenase-1 by Boswellic Acid Induces the Lipid Mediator Class Switch to Promote Resolution of Inflammation, Adv Sci (Weinh) (2022) e2205604.
[18] U. Siemoneit, A. Koeberle, A. Rossi, F. Dehm, M. Verhoff, S. Reckel, T.J. Maier, J. Jauch, H. Northoff, F. Bernhard, V. Doetsch, L. Sautebin, O. Werz, Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense, Br J Pharmacol 162(1) (2011) 147-62.
[19] S. Pace, K. Zhang, P.M. Jordan, R. Bilancia, W. Wang, F. Börner, R.K. Hofstetter, M. Potenza, C. Kretzer, J. Gerstmeier, Anti-inflammatory celastrol promotes a switch from leukotriene biosynthesis to formation of specialized pro-resolving lipid mediators, Pharmacological Research 167 (2021) 105556.
[20] C. Kretzer, P.M. Jordan, K.P. Meyer, D. Hoff, M. Werner, R.K. Hofstetter, A. Koeberle, A.C. Peralta, G. Viault, D. Seraphin, Natural chalcones elicit formation of specialized pro-resolving mediators and related 15-lipoxygenase products in human macrophages, Biochemical Pharmacology 195 (2022) 114825.
[21] T.T. Van Anh, A. Mostafa, Z. Rao, S. Pace, S. Schwaiger, C. Kretzer, V. Temml, C. Giesel, P.M. Jordan, R. Bilancia, C. Weinigel, S. Rummler, B. Waltenberger, T. Hung, A. Rossi, H. Stuppner, O. Werz, A. Koeberle, From Vietnamese plants to a biflavonoid that relieves inflammation by triggering the lipid mediator class switch to resolution, Acta Pharm Sin B 11(6) (2021) 1629-1647.
[22] C. Kretzer, P.M. Jordan, R. Bilancia, A. Rossi, T. Gur Maz, E. Banoglu, U.S. Schubert, O. Werz, Shifting the Biosynthesis of Leukotrienes Toward Specialized Pro-Resolving Mediators by the 5-Lipoxygenase-Activating Protein (FLAP) Antagonist BRP-201, J Inflamm Res 15 (2022) 911-925.
[23] P.M. Jordan, E. van Goethem, A.M. Muller, K. Hemmer, V. Gavioli, V. Baillif, Y. Burmeister, N. Krommelbein, M. Dubourdeau, B. Seilheimer, O. Werz, The Natural Combination Medicine Traumeel (Tr14) Improves Resolution of Inflammation by Promoting the Biosynthesis of Specialized Pro-Resolving Mediators, Pharmaceuticals (Basel) 14(11) (2021).
Autoren
leitet den Lehrstuhl für Pharmazeutische Medizinische Chemie am Institut für Pharmazie der Friedrich-Schiller-Universität Jena. Er erforscht die Grundlagen der Entzündungsregulation und sucht nach neuen pharmakologischen Strategien und Wirkstoffen zur Intervention mit entzündlichen Erkrankungen.