Health starts in the cell: numerous cellular processes and metabolic pathways, or their modulation, also play a role in the development of musculoskeletal diseases and complaints. This article summarises important basic scientific findings on the subject of nuclear magnetic resonance therapy (KSRT = Kernspinresonanz-Therapie, NMRT = nuclear magnetic resonance therapy, tNMR = therapeutic nuclear magnetic resonance, MBST®) for a better understanding from a medical perspective.
Particular mention should be made here of the fundamental scientific studies by Dr Steinecker-Frohnwieser et al. [1–5] and the work of the Innsbruck working group led by PD Dr Egg and Thöni Ph.D. [6–8]. The latter are very much involved in the scientific field of quantum biology, which is not yet widely known to the public. Researchers in this field are generally interested in, among other things, the effect of so-called low magnetic fields, as an aspect of physical therapies, on the body and its various processes. ‘Quantum biology has been an established field of research for decades, but in the public perception it still tends to occupy a niche existence,’ explained Egg, adding that ‘quantum biology deals with all processes in living beings that cannot be explained by classical physical laws, but only by principles of quantum mechanics’; for example, photosynthesis, the sense of direction and probably also the sense of smell and consciousness are based on quantum biological mechanisms [10].
Another important point in this context is that both a disruption of the cellular clocks or circadian rhythms (see Nobel Prize 2017) [11] and the HIF1 signalling pathways (see Nobel Prize 2019) [12] are now associated with a variety of diseases and have been described in our field, for example in connection with osteoarthritis and bone health, but also obesity [13–16]. Physical modulators would definitely be helpful in the contexts described, alongside the pharmacological approaches currently being researched, and would presumably also have fewer side effects due to their more physiological interaction. Previous data indicate that tNMR positively addresses or modulates precisely these pathways, which in turn have been proven to be interrelated, and could serve as a switch for the internal clock, so to speak. In summary, Egg et al. (in vitro) were able to show that tNMR influences these cellular clocks and the hypoxia regulator HIF1α in a novel way – without classic synchronisation – which, beyond its previous use, could also be used as a targeted therapeutic tool for hypoxia-related, i.e. ischaemic, diseases [6, 9].
Furthermore, tNMR can stabilise mitochondrial respiration under both normoxic and hypoxic conditions [7]. In addition, electromagnetic fields are known to stimulate the clock protein cryptochrome to modulate intracellular reactive oxygen species (ROS) via the quantum-based radical pair mechanism (RPM) in mammalian cells. Using synchronised NIH3T3 cells, for example, it has been shown that tNMR permanently alters cell metabolism under normoxia and hypoxia [7]. The latest publication also made it clear that tNMR and intermittent hypoxia control circadian cell rhythms via superoxide signals with time-dependent on/off effects and could thus form a potentially innovative basis for therapies for chronobiological and oxidative diseases [8, 9].
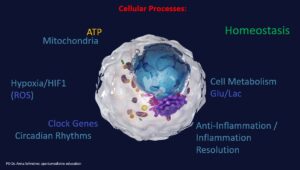
At the cellular level, numerous processes and metabolic pathways run simultaneously in parallel or consecutively. This is complex and makes it difficult, for example in the context of polypharmacy, to keep track of side effects and interactions once a certain number of medications are involved. Physical therapies, such as magnetic resonance therapy, aim to modulate many of these physiological processes in the sense of homeostasis and regeneration, which the body also strives for. The studies summarised in the article show a scientifically determined influence of magnetic resonance therapy on the exemplary influencing factors and processes.
The work from Vienna, in turn, showed in a rat model that NMRT has a positive effect on the survival of spinal ganglion neurons and neurite formation, which is primarily mediated by Schwann cell stimulation [4]. It also induces the secretion of ßNGF and pro-regenerative signalling factors, with ßNGF being considered the main factor for the neurotrophic/neuritogenic effects of NMRT in Schwann cells [5]. Nuclear magnetic resonance therapy could therefore also be considered a non-invasive, additive treatment option for peripheral nerve injuries. Further work by Steinecker-Frohnwieser using human chondrocytes showed that NMRT has both an anti-inflammatory effect (IL-1β ↓) that is clinically relevant, e.g., for pain reduction, and leads to a reduction in the expression of MMP13, which is involved in cartilage degradation, and influences the MAP kinase pathway and TGF-β [1]. Furthermore, chondrocytes are stabilized in vitro, which could help delay osteoarthritis-related cartilage damage, among other things by modulating cellular functions under inflammatory conditions through the adjustment of intracellular calcium (↑) or calcium release, NF-κB reduction, and intracellular ATP increase [2]. Interestingly, NMRT also altered microRNA (miR) profiles in vitro and modulated signalling pathways in human chondrocytes (HDAC, NAD+/NADH), i.e. NMRT was able to counteract the changes induced by IL-1β, for example by reducing catabolic effects and thereby reducing the inflammatory mechanisms in osteoarthritis by altering NF-kB signal transduction [3].
However, the older fundamental scientific work by Temiz-Artmann et al. [17,18] also made an important contribution to understanding the mode of action. The aim of the first study was to investigate whether MBST® magnetic resonance therapy has an effect on apoptosis, lifespan and proliferation of human chondrocytes and osteoblasts in vitro. No apoptosis or reduction in cell lifespan was observed. Instead, the number of chondrocytes/osteoblasts was significantly increased by 271/290% compared to the placebo group [17]. The results showed clear positive trends in cell growth rate after treatment with nuclear magnetic resonance, which correlates with clinical regeneration experiences in connection with some musculoskeletal applications. In a further study with primary human dermal fibroblasts, collagen in the extracellular matrix became more soluble in response to exposure of the cell structure to NMRT, i.e. less cross-linked, meaning it had a greater ability to bind GAG and water, which in clinical terms could be beneficial in compensating for age-related dehydration [18]. In addition to the protein expression effect of NMRT and clearly induced changes in (extra)cellular components, it was interestingly shown that NMRT is more effective in its combination of magnetic field and radio frequency than the individual components, which in turn was further elaborated and confirmed by current work from Innsbruck [19, 20].
The transfer or translation to the clinic and human patients is known to leave room for further influencing factors, so that—in addition to the need for more clinical MBST® data and studies—a steady increase in knowledge in all areas of physical therapy is also required in basic science. This correlates our treatments with the wealth of experience gained to date and can ultimately only improve them and—as is currently the case in the field of TPS, for example—also expand the areas of application [21].
Literature
- Steinecker-Frohnwieser B, Weigl L, Weberhofer G, Kullich W, Kress HG. The Influence of Nuclear Magnetic Resonance Therapy (NMRT) and Interleukin IL1-β Stimulation on Cal 78 Chondrosarcoma Cells and C28/I2 Chondrocytes. J Orthopedics Rheumatol. 2014;1(3): 9.
- Steinecker-Frohnwieser, B., Kullich, W., Mann, A., Kress, H. G., & Weigl, L. (2018). The therapeutic nuclear magnetic resonance changes the balance in intracellular calcium and reduces the interleukin-1β induced increase of NF-κB activity in chondrocytes. Clinical and experimental rheumatology, 36(2), 294–301.
- Steinecker-Frohnwieser, B., Lohberger, B., Eck, N., Mann, A., Kratschmann, C., Leithner, A., Kullich, W., & Weigl, L. (2021). Nuclear Magnetic Resonance Therapy Modulates the miRNA Profile in Human Primary OA Chondrocytes and Antagonizes Inflammation in Tc28/2a Cells. International journal of molecular sciences, 22(11), 5959. https://doi.org/10.3390/ijms22115959
- Mann, A., Steinecker-Frohnwieser, B., Naghilou, A., Millesi, F., Supper, P., Semmler, L., Wolf, S., Marinova, L., Weigl, L., Weiss, T., & Radtke, C. (2022). Nuclear Magnetic Resonance Treatment Accelerates the Regeneration of Dorsal Root Ganglion Neurons in vitro. Frontiers in cellular neuroscience, 16, 859545. https://doi.org/10.3389/fncel.2022.859545
- Rad, A., Weigl, L., Steinecker-Frohnwieser, B., Stadlmayr, S., Millesi, F., Haertinger, M., Borger, A., Supper, P., Semmler, L., Wolf, S., Naghilou, A., Weiss, T., Kress, H. G., & Radtke, C. (2024). Nuclear Magnetic Resonance Treatment Induces ßNGF Release from Schwann Cells and Enhances the Neurite Growth of Dorsal Root Ganglion Neurons In Vitro. Cells, 13(18), 1544. https://doi.org/10.3390/cells13181544
- Thoeni, V., Dimova, E. Y., Kietzmann, T., Usselman, R. J., & Egg, M. (2024). Therapeutic nuclear magnetic resonance and intermittent hypoxia trigger time dependent on/off effects in circadian clocks and confirm a central role of superoxide in cellular magnetic field effects. Redox biology, 72, 103152. https://doi.org/10.1016/j.redox.2024.103152
- Thöni, V., Mauracher, D., Ramalingam, A., Fiechtner, B., Sandbichler, A. M., & Egg, M. (2022). Quantum based effects of therapeutic nuclear magnetic resonance persistently reduce glycolysis. iScience, 25(12), 105536. https://doi.org/10.1016/j.isci.2022.105536
- Thöni, V., Oliva, R., Mauracher, D., & Egg, M. (2021). Therapeutic Nuclear Magnetic Resonance affects the core clock mechanism and associated Hypoxia-inducible factor-1. Chronobiology international, 38(8), 1120–1134. https://doi.org/10.1080/07420528.2021.1910288
- Egg, M., & Kietzmann, T. (2025). Little strokes fell big oaks: The use of weak magnetic fields and reactive oxygen species to fight cancer. Redox biology, 79, 103483. https://doi.org/10.1016/j.redox.2024.103483
- https://www.uibk.ac.at/de/newsroom/2022/durch-quantenbiologie-zu-neuen-therapieansatzen/
- https://www.aerzteblatt.de/archiv/nobelpreis-fuer-medizin-und-physiologie-warum-jeder-mensch-ueber-einen-regelrechten-uhrenladen-verfuegt-d5149f23-4ef6-4b8c-b3e2-038262d79df6
- https://www.aerzteblatt.de/archiv/nobelpreis-fuer-medizin-wenn-zellen-ausser-atem-kommen-3bded02a-58ff-454d-badf-f96bb794a606
- Guo H, Huang J, Liang Y, Wang D, Zhang H. Focusing on the hypoxia-inducible factor pathway: role, regulation, and therapy for osteoarthritis. Eur J Med Res. 2022;27(1):288. Published 2022 Dec 12. doi:10.1186/s40001-022-00926-2
- Yellowley CE, Genetos DC. Hypoxia Signaling in the Skeleton: Implications for Bone Health. Curr Osteoporos Rep. 2019;17(1):26-35. doi:10.1007/s11914-019-00500-6
- Winter EM, Kooijman S, Appelman-Dijkstra NM, Meijer OC, Rensen PC, Schilperoort M. Chronobiology and Chronotherapy of Osteoporosis. JBMR Plus. 2021;5(10):e10504. Published 2021 May 5. doi:10.1002/jbm4.10504
- Garaulet M, Gómez-Abellán P. Chronobiology and obesity. Nutr Hosp. 2013;28 Suppl 5:114-120. doi:10.3305/nh.2013.28.sup5.6926
- Temiz-Artmann A, Linder P, Kayser P, Digel I, Artmann GM, Lücker P. NMR in vitro effects on proliferation, apoptosis, and viability of human chondrocytes and osteoblasts. Methods Find Exp Clin Pharmacol. 2005;27(6):391-394. doi:10.1358/mf.2005.27.6.896831
- Digel I, Kurulgan E, Linder P, et al. Decrease in extracellular collagen crosslinking after NMR magnetic field application in skin fibroblasts. Med Biol Eng Comput. 2007;45(1):91-97. doi:10.1007/s11517-006-0144-z
- Viktoria Thöni, Abriana Buchter, Andreas Flarer, Justin Lampe, Cordula Schlegel, and Margit Egg „Quantitative differences in cellular effects between isolated sweep field and radiofrequency application in therapeutic nuclear magnetic resonance“, Proc. SPIE 13340, Quantum Effects and Measurement Techniques in Biology and Biophotonics II, 1334006 (21 March 2025); https://doi.org/10.1117/12.3051909
- Viktoria Thöni, Abriana Buchter, Andreas Flarer, Justin Lampe, Cordula Schlegel, and Margit Egg „Understanding therapeutic nuclear magnetic resonance (tNMR): splitting of components indicates its unique efficacy“, Proc. SPIE 13340, Quantum Effects and Measurement Techniques in Biology and Biophotonics II, 133400E (21 March 2025); https://doi.org/10.1117/12.3056086
- Karsten Knobloch. Novel Transcranial Pulse Stimulation (TPS) in Football-Related Concussion – A Pilot Case Series. Journal of Orthopedics and Sports Medicine. 7 (2025): 373-378.
Autoren
ist Fachärztin für Orthopädie und Unfallchirurgie. Sie war jahrelang an renommierten Kliniken tätig, absolvierte ein Research Fellowship in den USA und war u.a. auch Sektionsleiterin Wissenschaft des Jungen Forums O&U. Sie wechselte dann in die Industrie und ist aktuell als Chief Medical Officer bei der MedTec Medizintechnik GmbH tätig. Sie lehrt weiterhin an der Eberhard Karls Universität Tübingen bzw. der dortigen Orthopädischen Universitätsklinik und ist Dozentin der AKAD.